Human iPSC-based cardiac microphysiological system for drug screening applications
- PMID: 25748532
- PMCID: PMC4352848
- DOI: 10.1038/srep08883
Human iPSC-based cardiac microphysiological system for drug screening applications
Abstract
Drug discovery and development are hampered by high failure rates attributed to the reliance on non-human animal models employed during safety and efficacy testing. A fundamental problem in this inefficient process is that non-human animal models cannot adequately represent human biology. Thus, there is an urgent need for high-content in vitro systems that can better predict drug-induced toxicity. Systems that predict cardiotoxicity are of uppermost significance, as approximately one third of safety-based pharmaceutical withdrawals are due to cardiotoxicty. Here, we present a cardiac microphysiological system (MPS) with the attributes required for an ideal in vitro system to predict cardiotoxicity: i) cells with a human genetic background; ii) physiologically relevant tissue structure (e.g. aligned cells); iii) computationally predictable perfusion mimicking human vasculature; and, iv) multiple modes of analysis (e.g. biological, electrophysiological, and physiological). Our MPS is able to keep human induced pluripotent stem cell derived cardiac tissue viable and functional over multiple weeks. Pharmacological studies using the cardiac MPS show half maximal inhibitory/effective concentration values (IC₅₀/EC₅₀) that are more consistent with the data on tissue scale references compared to cellular scale studies. We anticipate the widespread adoption of MPSs for drug screening and disease modeling.
Figures
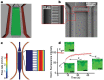
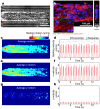
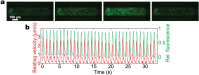

References
-
- Herper M. The Cost Of Creating A New Drug Now $5 Billion, Pushing Big Pharma To Change <http://www.forbes.com/sites/matthewherper/2013/08/11/how-the-staggering-...> (2013).
-
- Paul S. M. et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nature reviews. Drug discovery 9, 203–214 (2010). - PubMed
-
- Scott C. W., Peters M. F. & Dragan Y. P. Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol Lett 219, 49–58 (2013). - PubMed
-
- Chi K. R. Revolution dawning in cardiotoxicity testing. Nature reviews. Drug discovery 12, 565–567 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

