The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells?
- PMID: 25748930
- PMCID: PMC4355577
- DOI: 10.1016/j.stem.2015.02.015
The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells?
Abstract
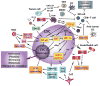
Cancer stem cells (CSCs) are tumor cells that have the principal properties of self-renewal, clonal tumor initiation capacity, and clonal long-term repopulation potential. CSCs reside in niches, which are anatomically distinct regions within the tumor microenvironment. These niches maintain the principle properties of CSCs, preserve their phenotypic plasticity, protect them from the immune system, and facilitate their metastatic potential. In this perspective, we focus on the CSC niche and discuss its contribution to tumor initiation and progression. Since CSCs survive many commonly employed cancer therapies, we examine the prospects of targeting the niche components as preferable therapeutic targets.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294:139–46. - PubMed
-
- Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, et al. TGF-β Receptor Inhibitors Target the CD44high/Id1high Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–668. - PubMed
-
- Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

