Retinoic acid-induced IgG production in TLR-activated human primary B cells involves ULK1-mediated autophagy
- PMID: 25749095
- PMCID: PMC4502696
- DOI: 10.1080/15548627.2015.1009797
Retinoic acid-induced IgG production in TLR-activated human primary B cells involves ULK1-mediated autophagy
Abstract
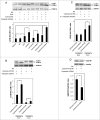
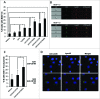
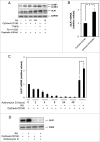
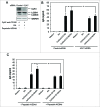
In the present study we have established a vital role of autophagy in retinoic acid (RA)-induced differentiation of toll-like receptor (TLR)-stimulated human B cells into Ig-secreting cells. Thus, RA enhanced autophagy in TLR9- and CD180-stimulated peripheral blood B cells, as revealed by increased levels of the autophagosomal marker LC3B-II, enhanced colocalization between LC3B and the lysosomal marker Lyso-ID, by a larger percentage of cells with more than 5 characteristic LC3B puncta, and by the concomitant reduction in the level of SQSTM1/p62. Furthermore, RA induced expression of the autophagy-inducing protein ULK1 at the transcriptional level, in a process that required the retinoic acid receptor RAR. By inhibiting autophagy with specific inhibitors or by knocking down ULK1 by siRNA, the RA-stimulated IgG production in TLR9- and CD180-mediated cells was markedly reduced. We propose that the identified prominent role of autophagy in RA-mediated IgG-production in normal human B cells provides a novel mechanism whereby vitamin A exerts its important functions in the immune system.
Keywords: ATG, autophagy-related; B lymphocytes; BDS, bright detail similarity; CD180; CD180, CD180 molecule; CVID, common variable immune deficiency; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; Ig, immunoglobulin; MAP1LC3B/LC3B, microtubule-associated protein 1 light chain 3 β; MTOR, mechanistic target of rapamycin (serine/threonine kinase); PAMP, pathogen-associated molecular pattern, PML/RARA, promyelocytic leukemia/ retinoic acid receptor α; RA, all-trans retinoic acid; RAR, retinoic acid receptor; RP105; SQSTM1, sequestosome 1; TLR, toll-like receptor; TLR9; ULK1; ULK1, unc-51 like autophagy activating kinase 1; antibody secretion; autophagy; plasma cell differentiation; retinoic acid.
Figures


Similar articles
-
TLR9-signaling is required for turning retinoic acid into a potent stimulator of RP105 (CD180)-mediated proliferation and IgG synthesis in human memory B cells.Cell Immunol. 2012 Sep;279(1):87-95. doi: 10.1016/j.cellimm.2012.09.003. Epub 2012 Sep 19. Cell Immunol. 2012. PMID: 23103284
-
Retinoic acid improves defective TLR9/RP105-induced immune responses in common variable immunodeficiency-derived B cells.J Immunol. 2013 Oct 1;191(7):3624-33. doi: 10.4049/jimmunol.1300213. Epub 2013 Sep 4. J Immunol. 2013. PMID: 24006462
-
IRF4 Is a Critical Gene in Retinoic Acid-Mediated Plasma Cell Formation and Is Deregulated in Common Variable Immunodeficiency-Derived B Cells.J Immunol. 2015 Sep 15;195(6):2601-11. doi: 10.4049/jimmunol.1500250. Epub 2015 Aug 14. J Immunol. 2015. PMID: 26276871
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Long non-coding RNAs involved in autophagy regulation.Cell Death Dis. 2017 Oct 5;8(10):e3073. doi: 10.1038/cddis.2017.464. Cell Death Dis. 2017. PMID: 28981093 Free PMC article. Review.
-
The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway.Cell Death Differ. 2017 Feb;24(2):212-224. doi: 10.1038/cdd.2016.111. Epub 2016 Oct 14. Cell Death Differ. 2017. PMID: 27740626 Free PMC article.
-
cAMP-mediated autophagy inhibits DNA damage-induced death of leukemia cells independent of p53.Oncotarget. 2018 Jul 13;9(54):30434-30449. doi: 10.18632/oncotarget.25758. eCollection 2018 Jul 13. Oncotarget. 2018. PMID: 30100998 Free PMC article.
-
Roles of Autophagy and Autophagy-Related Proteins in Antifungal Immunity.Front Immunol. 2016 Feb 18;7:47. doi: 10.3389/fimmu.2016.00047. eCollection 2016. Front Immunol. 2016. PMID: 26925060 Free PMC article. Review.
-
Intracellular alpha-fetoprotein interferes with all-trans retinoic acid induced ATG7 expression and autophagy in hepatocellular carcinoma cells.Sci Rep. 2021 Jan 25;11(1):2146. doi: 10.1038/s41598-021-81678-7. Sci Rep. 2021. PMID: 33495541 Free PMC article.
References
-
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 2007; 8:931-7; PMID:17712358; http://dx.doi.org/10.1038/nrm2245 - DOI - PubMed
-
- Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev 2011; 240:92-104; PMID:21349088; http://dx.doi.org/10.1111/j.1600-065X.2010.00995.x - DOI - PMC - PubMed
-
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011; 469:323-35; PMID:21248839; http://dx.doi.org/10.1038/nature09782 - DOI - PMC - PubMed
-
- Chaturvedi A, Pierce SK. Autophagy in immune cell regulation and dysregulation. Curr Allergy Asthma Rep 2009; 9:341-6; PMID:19671376; http://dx.doi.org/10.1007/s11882-009-0050-1 - DOI - PubMed
-
- McLeod IX, He Y. Roles of autophagy in lymphocytes: reflections and directions. Cell Mol Immunol 2010; 7:104-7; PMID:20118969; http://dx.doi.org/10.1038/cmi.2009.115 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
