Irisin - a myth rather than an exercise-inducible myokine
- PMID: 25749243
- PMCID: PMC4352853
- DOI: 10.1038/srep08889
Irisin - a myth rather than an exercise-inducible myokine
Abstract
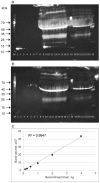
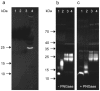
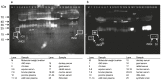
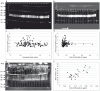
The myokine irisin is supposed to be cleaved from a transmembrane precursor, FNDC5 (fibronectin type III domain containing 5), and to mediate beneficial effects of exercise on human metabolism. However, evidence for irisin circulating in blood is largely based on commercial ELISA kits which are based on polyclonal antibodies (pAbs) not previously tested for cross-reacting serum proteins. We have analyzed four commercial pAbs by Western blotting, which revealed prominent cross-reactivity with non-specific proteins in human and animal sera. Using recombinant glycosylated and non-glycosylated irisin as positive controls, we found no immune-reactive bands of the expected size in any biological samples. A FNDC5 signature was identified at ~20 kDa by mass spectrometry in human serum but was not detected by the commercial pAbs tested. Our results call into question all previous data obtained with commercial ELISA kits for irisin, and provide evidence against a physiological role for irisin in humans and other species.
Figures

References
-
- Timmons J. A., Baar K., Davidsen P. K. & Atherton P. J. Is irisin a human exercise gene? Nature 488, E9–10 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

