RIG-I in RNA virus recognition
- PMID: 25749629
- PMCID: PMC4424084
- DOI: 10.1016/j.virol.2015.02.017
RIG-I in RNA virus recognition
Abstract
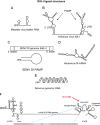
Antiviral immunity is initiated upon host recognition of viral products via non-self molecular patterns known as pathogen-associated molecular patterns (PAMPs). Such recognition initiates signaling cascades that induce intracellular innate immune defenses and an inflammatory response that facilitates development of the acquired immune response. The retinoic acid-inducible gene I (RIG-I) and the RIG-I-like receptor (RLR) protein family are key cytoplasmic pathogen recognition receptors that are implicated in the recognition of viruses across genera and virus families, including functioning as major sensors of RNA viruses, and promoting recognition of some DNA viruses. RIG-I, the charter member of the RLR family, is activated upon binding to PAMP RNA. Activated RIG-I signals by interacting with the adapter protein MAVS leading to a signaling cascade that activates the transcription factors IRF3 and NF-κB. These actions induce the expression of antiviral gene products and the production of type I and III interferons that lead to an antiviral state in the infected cell and surrounding tissue. RIG-I signaling is essential for the control of infection by many RNA viruses. Recently, RIG-I crosstalk with other pathogen recognition receptors and components of the inflammasome has been described. In this review, we discuss the current knowledge regarding the role of RIG-I in recognition of a variety of virus families and its role in programming the adaptive immune response through cross-talk with parallel arms of the innate immune system, including how RIG-I can be leveraged for antiviral therapy.
Keywords: Infection; Innate immunity; Pathogen-associated molecular pattern; RIG-I; RIG-I-like receptor; RNA virus.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- U54 AI081680/AI/NIAID NIH HHS/United States
- U19 AI083019/AI/NIAID NIH HHS/United States
- AI88778/AI/NIAID NIH HHS/United States
- R44 AI081335/AI/NIAID NIH HHS/United States
- R01 DA024563/DA/NIDA NIH HHS/United States
- R01 AI074973/AI/NIAID NIH HHS/United States
- R56 AI060389/AI/NIAID NIH HHS/United States
- AI083019/AI/NIAID NIH HHS/United States
- U19 AI088778/AI/NIAID NIH HHS/United States
- R01 AI098943/AI/NIAID NIH HHS/United States
- R01 AI104002/AI/NIAID NIH HHS/United States
- U19 AI100625/AI/NIAID NIH HHS/United States
- AI104002-01/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

