Carbopol-incorporated thermoreversible gel for intranasal drug delivery
- PMID: 25749681
- PMCID: PMC6272239
- DOI: 10.3390/molecules20034124
Carbopol-incorporated thermoreversible gel for intranasal drug delivery
Abstract
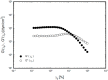
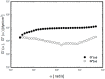
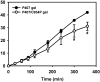
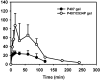
The present study describes the preparation and evaluation of a poloxamer 407 (P407)-based thermoreversible gel using Carbopol 934P (C934P) as a mucoadhesive polymer and hydroxypropyl-β-cyclodextrin (HP-β-CD) for enhancing the aqueous solubility and intranasal absorption of fexofenadine hydrochloride (FXD HCl). The prepared gels were characterized by gelation temperature, viscoelasticity, and drug release profile. Thermoreversibility of P407/C934P gel was demonstrated by rheological studies. The incorporation of carbopol into P407 gel also reduced the amounts of drug released from the gel formulations (p < 0.05). In vivo pharmacokinetic results of the prepared gel formulations in rabbits (at 0.5 mg/kg dose) showed that the relative bioavailability of drug from P407/C934P gel was 11.3 and 2.7-fold higher than those of drug solution and P407 gel group, respectively. These findings suggested that developed thermoreversible gels could be used as promising dosage forms to improve intranasal drug absorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

