Evaluation of Trastuzumab Anti-Tumor Efficacy and its Correlation with HER-2 Status in Patient-Derived Gastric Adenocarcinoma Xenograft Models
- PMID: 25749810
- PMCID: PMC4550655
- DOI: 10.1007/s12253-015-9909-8
Evaluation of Trastuzumab Anti-Tumor Efficacy and its Correlation with HER-2 Status in Patient-Derived Gastric Adenocarcinoma Xenograft Models
Abstract
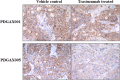
The aim of the study was to investigate trastuzumab anti-tumor efficacy and its correlation with HER-2 status in primary xenograft models derived from Chinese patients with gastric adenocarcinoma. Patient-derived gastric adenocarcinoma xenograft (PDGAX) mouse models were firstly generated by implanting gastric adenocarcinoma tissues from patients into immune deficient mice. A high degree of histological and molecular similarity between the PDGAX mouse models and their corresponding patients' gastric adenocarcinoma tissues was shown by pathological observation, HER-2 expression, HER-2 gene copy number, and mutation detection. Based on Hoffmann's criteria in gastric cancer, three models (PDGAX001, PDGAX003 and PDGAX005) were defined as HER-2 positive with fluorescence in situ hybridization (FISH) amplification or immunohistochemistry (IHC) 2+/ 3+, while two models (PDGAX002, PDGAX004) were defined as HER-2 negative. Upon trastuzumab treatment, significant tumor regression (105 % TGI) was observed in model PDGAX005 (TP53 wt), while moderate sensitivity (26 % TGI) was observed in PDGAX003, and resistance was observed in PDGAX001, 002 and 004. A significant increase in HER-2 gene copy number was only observed in PDGAX005 (TP53 wt). Interestingly, trastuzumab showed no efficacy in PDGAX001 (HER2 IHC 3+ and FISH amplification, but with mutant TP53). Consistent with this finding, phosphor-HER2 modulation by trastuzumab was observed in model PDGAX005, but not in PDGAX001.
Conflict of interest statement
The authors with AstraZeneca affiliation are full time employees and/or have stock in AstraZeneca. AstraZeneca sponsored this study.
Figures





Similar articles
-
Patient-Derived Gastric Carcinoma Xenograft Mouse Models Faithfully Represent Human Tumor Molecular Diversity.PLoS One. 2015 Jul 28;10(7):e0134493. doi: 10.1371/journal.pone.0134493. eCollection 2015. PLoS One. 2015. PMID: 26217940 Free PMC article.
-
Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer.Cancer Lett. 2011 Jul 28;306(2):171-9. doi: 10.1016/j.canlet.2011.03.002. Epub 2011 Apr 1. Cancer Lett. 2011. PMID: 21458915
-
Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma.Virchows Arch. 2014 Aug;465(2):145-54. doi: 10.1007/s00428-014-1597-3. Epub 2014 Jun 3. Virchows Arch. 2014. PMID: 24889042
-
HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice.World J Gastroenterol. 2016 Jul 14;22(26):5879-87. doi: 10.3748/wjg.v22.i26.5879. World J Gastroenterol. 2016. PMID: 27468182 Free PMC article. Review.
-
HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications.Arch Pathol Lab Med. 2012 Jun;136(6):691-7. doi: 10.5858/arpa.2011-0168-RS. Arch Pathol Lab Med. 2012. PMID: 22646280 Review.
Cited by
-
ErbB Family Signalling: A Paradigm for Oncogene Addiction and Personalized Oncology.Cancers (Basel). 2017 Apr 12;9(4):33. doi: 10.3390/cancers9040033. Cancers (Basel). 2017. PMID: 28417948 Free PMC article. Review.
-
Advances and challenges in the treatment of esophageal cancer.Acta Pharm Sin B. 2021 Nov;11(11):3379-3392. doi: 10.1016/j.apsb.2021.03.008. Epub 2021 Mar 9. Acta Pharm Sin B. 2021. PMID: 34900524 Free PMC article. Review.
-
Establishment of Novel Gastric Cancer Patient-Derived Xenografts and Cell Lines: Pathological Comparison between Primary Tumor, Patient-Derived, and Cell-Line Derived Xenografts.Cells. 2019 Jun 14;8(6):585. doi: 10.3390/cells8060585. Cells. 2019. PMID: 31207870 Free PMC article.
-
Patient-Derived Gastric Carcinoma Xenograft Mouse Models Faithfully Represent Human Tumor Molecular Diversity.PLoS One. 2015 Jul 28;10(7):e0134493. doi: 10.1371/journal.pone.0134493. eCollection 2015. PLoS One. 2015. PMID: 26217940 Free PMC article.
References
-
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8:437–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

