Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs
- PMID: 25749929
- PMCID: PMC4407720
- DOI: 10.1124/jpet.114.220368
Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs
Abstract
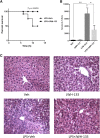
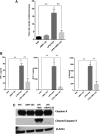
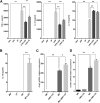
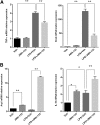
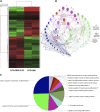
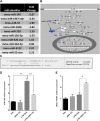
Acute liver failure (ALF) is a potentially life-threatening disorder without any effective treatment strategies. d-Galactosamine (GalN)/lipopolysaccharide (LPS)-induced ALF is a widely used animal model to identify novel hepato-protective agents. In the present study, we investigated the potential of a cannabinoid receptor 2 (CB2) agonist, JWH-133 [(6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran], in the amelioration of GalN/LPS-induced ALF. JWH-133 treatment protected the mice from ALF-associated mortality, mitigated alanine transaminase and proinflammatory cytokines, suppressed histopathological and apoptotic liver damage, and reduced liver infiltration of mononuclear cells (MNCs). Furthermore, JWH-133 pretreatment of M1/M2-polarized macrophages significantly increased the secretion of anti-inflammatory cytokine interleukin-10 (IL-10) in M1 macrophages and potentiated the expression of M2 markers in M2-polarized macrophages. In vivo, JWH-133 treatment also suppressed ALF-triggered expression of M1 markers in liver MNCs, while increasing the expression of M2 markers such as Arg1 and IL-10. microRNA (miR) microarray analysis revealed that JWH-133 treatment altered the expression of only a few miRs in the liver MNCs. Gene ontology analysis of the targets of miRs suggested that Toll-like receptor (TLR) signaling was among the most significantly targeted cellular pathways. Among the altered miRs, miR-145 was found to be the most significantly decreased. This finding correlated with concurrent upregulated expression of its predicted target gene, interleukin-1 receptor-associated kinase 3, a negative regulator of TLR4 signaling. Together, these data are the first to demonstrate that CB2 activation attenuates GalN/LPS-induced ALF by inducing an M1 to M2 shift in macrophages and by regulating the expression of unique miRs that target key molecules involved in the TLR4 pathway.
U.S. Government work not protected by U.S. copyright.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

