Down-regulation of ZmEXPB6 (Zea mays β-expansin 6) protein is correlated with salt-mediated growth reduction in the leaves of Z. mays L
- PMID: 25750129
- PMCID: PMC4416831
- DOI: 10.1074/jbc.M114.619718
Down-regulation of ZmEXPB6 (Zea mays β-expansin 6) protein is correlated with salt-mediated growth reduction in the leaves of Z. mays L
Abstract
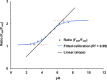
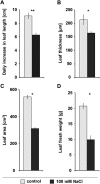
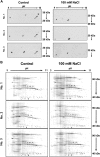
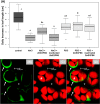
The salt-sensitive crop Zea mays L. shows a rapid leaf growth reduction upon NaCl stress. There is increasing evidence that salinity impairs the ability of the cell walls to expand, ultimately inhibiting growth. Wall-loosening is a prerequisite for cell wall expansion, a process that is under the control of cell wall-located expansin proteins. In this study the abundance of those proteins was analyzed against salt stress using gel-based two-dimensional proteomics and two-dimensional Western blotting. Results show that ZmEXPB6 (Z. mays β-expansin 6) protein is lacking in growth-inhibited leaves of salt-stressed maize. Of note, the exogenous application of heterologously expressed and metal-chelate-affinity chromatography-purified ZmEXPB6 on growth-reduced leaves that lack native ZmEXPB6 under NaCl stress partially restored leaf growth. In vitro assays on frozen-thawed leaf sections revealed that recombinant ZmEXPB6 acts on the capacity of the walls to extend. Our results identify expansins as a factor that partially restores leaf growth of maize in saline environments.
Keywords: Cell Growth; Growth Inhibition; Linear Variable Differential Transducer; Plant; Plant Biochemistry; Plant Cell Wall; Real-time Fluorescence Ratio Imaging; Salinity; Stress; Zea mays L.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Rozema J., Flowers T. (2008) Crops for a salinized world. Science 322, 1478–1480 - PubMed
-
- Cushman J., Tester M. (2010) Special issue on drought and salinity stress. Plant Cell Environ. 4, 453–669
-
- Hütsch B. W., Saqib M., Osthushenrich T., Schubert S. (2014) Invertase activity limits grain yield of maize under salt stress. J. Plant Nutr. Soil Sci. 177, 278–286
-
- Bernstein N., Silk W. K., Läuchli A. (1993) Growth and development of sorghum leaves under conditions of NaCl stress: spatial and temporal aspects of leaf growth inhibition. Planta 191, 433–439
-
- Munns R., Tester M. (2008) Mechanism of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

