Neospora caninum Recruits Host Cell Structures to Its Parasitophorous Vacuole and Salvages Lipids from Organelles
- PMID: 25750213
- PMCID: PMC4421005
- DOI: 10.1128/EC.00262-14
Neospora caninum Recruits Host Cell Structures to Its Parasitophorous Vacuole and Salvages Lipids from Organelles
Abstract
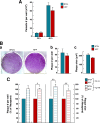
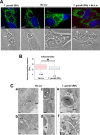
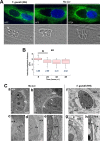
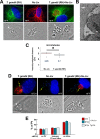
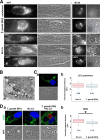
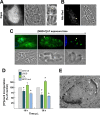
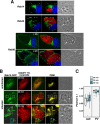
Toxoplasma gondii and Neospora caninum, which cause the diseases toxoplasmosis and neosporosis, respectively, are two closely related apicomplexan parasites. They have similar heteroxenous life cycles and conserved genomes and share many metabolic features. Despite these similarities, T. gondii and N. caninum differ in their transmission strategies and zoonotic potential. Comparative analyses of the two parasites are important to identify the unique biological features that underlie the basis of host preference and pathogenicity. T. gondii and N. caninum are obligate intravacuolar parasites; in contrast to T. gondii, events that occur during N. caninum infection remain largely uncharacterized. We examined the capability of N. caninum (Liverpool isolate) to interact with host organelles and scavenge nutrients in comparison to that of T. gondii (RH strain). N. caninum reorganizes the host microtubular cytoskeleton and attracts endoplasmic reticulum (ER), mitochondria, lysosomes, multivesicular bodies, and Golgi vesicles to its vacuole though with some notable differences from T. gondii. For example, the host ER gathers around the N. caninum parasitophorous vacuole (PV) but does not physically associate with the vacuolar membrane; the host Golgi apparatus surrounds the N. caninum PV but does not fragment into ministacks. N. caninum relies on plasma lipoproteins and scavenges cholesterol from NPC1-containing endocytic organelles. This parasite salvages sphingolipids from host Golgi Rab14 vesicles that it sequesters into its vacuole. Our data highlight a remarkable degree of conservation in the intracellular infection program of N. caninum and T. gondii. The minor differences between the two parasites related to the recruitment and rearrangement of host organelles around their vacuoles likely reflect divergent evolutionary paths.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Dubey JP, Barr BC, Barta JR, Bjerkas I, Bjorkman C, Blaqburn BJ, Bowman DD, Buxton D, Ellis JT, Gottstein B, Hemphill A, Hill DE, Howe DK, Jenkins MC, Kobayashi Y, Koudela B, Marsh AE, Mattsson JG, McAllister MM, Modrý D, Omata Y, Sibley LD, Speer CA, Trees AJ, Uggla A, Upton SJ, Williams DJ, Lindsay DS. 2002. Redescription of Neospora caninum and its differentiation from related coccidia. Int J Parasitol 32:929–946. doi:10.1016/S0020-7519(02)00094-2. - DOI - PubMed
-
- Reid AJ, Vermont SJ, Cotton JA, Harris D, Hill-Cawthorne GA, Könen-Waisman S, Latham SM, Mourier T, Norton R, Quail MA, Sanders M, Shanmugam D, Sohal A, Wasmuth JD, Brunk B, Grigg ME, Howard JC, Parkinson J, Roos DS, Trees AJ, Berriman M, Pain A, Wastling JM. 2012. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog 8:e1002567. doi:10.1371/journal.ppat.1002567. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

