Effectiveness and tolerability of second-line treatment with vildagliptin versus other oral drugs for type 2 diabetes in a real-world setting in the Middle East: results from the EDGE study
- PMID: 25750538
- PMCID: PMC4348128
- DOI: 10.2147/VHRM.S73703
Effectiveness and tolerability of second-line treatment with vildagliptin versus other oral drugs for type 2 diabetes in a real-world setting in the Middle East: results from the EDGE study
Abstract
Background: Type 2 diabetes mellitus (T2DM) is a chronic progressive disease that requires treatment intensification with antihyperglycemic agents due to progressive deterioration of β-cell function. A large observational study of 45,868 patients with T2DM across 27 countries (EDGE) assessed the effectiveness and safety of vildagliptin as add-on to other oral antidiabetic drugs (OADs) versus other comparator OAD combinations. Here, we present results from the Middle East countries (Bahrain, Jordan, Kuwait, Lebanon, Oman, Palestine, and the United Arab Emirates).
Methods: Patients inadequately controlled with OAD monotherapy were eligible after the add-on treatment was chosen by the physician based on clinical judgment and patient need. Patients were assigned to either vildagliptin or comparator OADs (sulfonylureas, thiazolidinediones, glinides, α-glucosidase inhibitors, or metformin, except incretin-based therapies) based on the add-on therapy. The primary endpoint was the proportion of patients achieving a glycated hemoglobin (HbA1c) reduction of >0.3% without peripheral edema, hypoglycemia, discontinuation due to a gastrointestinal event, or weight gain≥5%. One of the secondary endpoints was the proportion of patients achieving HbA1c<7% without hypoglycemia or weight gain. Change in HbA1c from baseline to study endpoint and safety were also assessed.
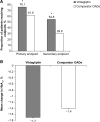
Results: Of the 4,780 patients enrolled in the Middle East, 2,513 received vildagliptin and 2,267 received other OADs. Overall, the mean (±standard deviation) age at baseline was 52.1±10.2 years, mean HbA1c was 8.5%±1.3%, and mean T2DM duration was 4.2±4.0 years. The proportion of patients achieving the primary (76.1% versus 61.6%, P<0.0001) and secondary (54.8% versus 29.9%, P<0.0001) endpoints was higher with vildagliptin than with the comparator OADs. The unadjusted odds ratios for the primary and secondary endpoints were 1.98 (95% confidence interval 1.75-2.25) and 2.8 (95% confidence interval 2.5-3.2), respectively, in favor of vildagliptin. Vildagliptin achieved a numerically greater reduction in HbA1c (1.7%) from baseline versus comparator OADs (1.4%). The overall incidence of adverse events was comparable between studied cohorts.
Conclusion: In real life, treatment with vildagliptin was associated with a higher proportion of patients with T2DM achieving better glycemic control without tolerability issues in the Middle East.
Keywords: Middle East; dipeptidyl peptidase-4; oral antidiabetic drugs; real world; type 2 diabetes mellitus; vildagliptin.
Figures

References
-
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. - PubMed
-
- Majeed A, El-Sayed AA, Khoja T, Alshamsan R, Millett C, Rawaf S. Diabetes in the Middle-East and North Africa: an update for 2013 for the IDF Diabetes Atlas. Diabetes Res Clin Pract. 2014;103:218–222. - PubMed
-
- Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–1255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

