Structure and mechanism of ABC transporters
- PMID: 25750732
- PMCID: PMC4338842
- DOI: 10.12703/P7-14
Structure and mechanism of ABC transporters
Abstract
All living organisms depend on primary and secondary membrane transport for the supply of external nutrients and removal or sequestration of unwanted (toxic) compounds. Due to the chemical diversity of cellular molecules, it comes as no surprise that a significant part of the proteome is dedicated to the active transport of cargo across the plasma membrane or the membranes of subcellular organelles. Transport against a chemical gradient can be driven by, for example, the free energy change associated with ATP hydrolysis (primary transport), or facilitated by the potential energy of the chemical gradient of another molecule (secondary transport). Primary transporters include the rotary motor ATPases (F-, A-, and V-ATPases), P-type ATPases and a large family of integral membrane proteins referred to as "ABC" (ATP binding cassette) transporters. ABC transporters are widespread in all forms of life and are characterized by two nucleotide-binding domains (NBD) and two transmembrane domains (TMDs). ATP hydrolysis on the NBD drives conformational changes in the TMD, resulting in alternating access from inside and outside of the cell for unidirectional transport across the lipid bilayer. Common to all ABC transporters is a signature sequence or motif, LSGGQ, that is involved in nucleotide binding. Both importing and exporting ABC transporters are found in bacteria, whereas the majority of eukaryotic family members function in the direction of export. Recent progress with the X-ray crystal structure determination of a variety of bacterial and eukaryotic ABC transporters has helped to advance our understanding of the ATP hydrolysis-driven transport mechanism but has also illustrated the large structural and functional diversity within the family.
Figures
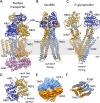

References
-
- Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science (New York, N.Y.) 1997;277:1453–62. doi: 10.1126/science.277.5331.1453. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

