Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle
- PMID: 25750996
- PMCID: PMC4353621
- DOI: 10.1371/journal.pone.0119587
Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle
Abstract
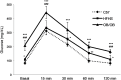
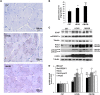
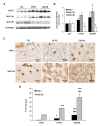
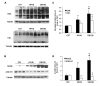
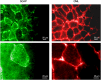
Aim of this study was to investigate whether advanced glycation end-products (AGEs) accumulate in skeletal myofibers of two different animal models of diabesity and whether this accumulation could be associated to myosteatosis. Male C57Bl/6j mice and leptin-deficient ob/ob mice were divided into three groups and underwent 15 weeks of dietary manipulation: standard diet-fed C57 group (C57, n = 10), high-fat high-sugar diet-fed C57 group (HFHS, n = 10), and standard diet-fed ob/ob group (OB/OB, n = 8). HFHS mice and OB/OB mice developed glycometabolic abnormalities in association with decreased mass of the gastrocnemius muscle, fast-to-slow transition of muscle fibers, and lipid accumulation (that occurred preferentially in slow compared to fast fibers). Moreover, we found in muscle fibers of HFHS and OB/OB mice accumulation of AGEs that was preferential for the lipid-accumulating cells, increased expression of the lipogenic pathway SCAP/SREBP, and co-localisation between AGEs and SCAP-(hyper)expressing cells (suggestive for SCAP glycosylation). The increased expression of the SCAP/SREBP lipogenic pathway in muscle fibers is a possible mechanism underlying lipid accumulation and linking myosteatosis to muscle fiber atrophy and fast-to-slow transition that occur in response to diabesity.
Conflict of interest statement
Figures



References
-
- Bifulco M, Caruso MG. From the gastronomic revolution to the new globesity epidemic. J Am Diet Assoc. 2007;107: 2058–2060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

