CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage
- PMID: 25751061
- PMCID: PMC4396484
- DOI: 10.1172/JCI78578
CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage
Abstract
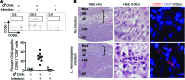
Mammalian pregnancy requires protection against immunological rejection of the developing fetus bearing discordant paternal antigens. Immune evasion in this developmental context entails silenced expression of chemoattractant proteins (chemokines), thereby preventing harmful immune cells from penetrating the maternal-fetal interface. Here, we demonstrate that fetal wastage triggered by prenatal Listeria monocytogenes infection is driven by placental recruitment of CXCL9-producing inflammatory neutrophils and macrophages that promote infiltration of fetal-specific T cells into the decidua. Maternal CD8+ T cells with fetal specificity upregulated expression of the chemokine receptor CXCR3 and, together with neutrophils and macrophages, were essential for L. monocytogenes-induced fetal resorption. Conversely, decidual accumulation of maternal T cells with fetal specificity and fetal wastage were extinguished by CXCR3 blockade or in CXCR3-deficient mice. Remarkably, protection against fetal wastage and in utero L. monocytogenes invasion was maintained even when CXCR3 neutralization was initiated after infection, and this protective effect extended to fetal resorption triggered by partial ablation of immune-suppressive maternal Tregs, which expand during pregnancy to sustain fetal tolerance. Together, our results indicate that functionally overriding chemokine silencing at the maternal-fetal interface promotes the pathogenesis of prenatal infection and suggest that therapeutically reinforcing this pathway represents a universal approach for mitigating immune-mediated pregnancy complications.
Figures









Comment in
-
Immunology: Stillbirth prevented by signal blockade.Nature. 2015 Apr 30;520(7549):627-8. doi: 10.1038/520627a. Nature. 2015. PMID: 25925473 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

