KIM-1-mediated phagocytosis reduces acute injury to the kidney
- PMID: 25751064
- PMCID: PMC4396492
- DOI: 10.1172/JCI75417
KIM-1-mediated phagocytosis reduces acute injury to the kidney
Abstract
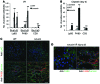
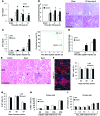
Kidney injury molecule 1 (KIM-1, also known as TIM-1) is markedly upregulated in the proximal tubule after injury and is maladaptive when chronically expressed. Here, we determined that early in the injury process, however, KIM-1 expression is antiinflammatory due to its mediation of phagocytic processes in tubule cells. Using various models of acute kidney injury (AKI) and mice expressing mutant forms of KIM-1, we demonstrated a mucin domain-dependent protective effect of epithelial KIM-1 expression that involves downregulation of innate immunity. Deletion of the mucin domain markedly impaired KIM-1-mediated phagocytic function, resulting in increased proinflammatory cytokine production, decreased antiinflammatory growth factor secretion by proximal epithelial cells, and a subsequent increase in tissue macrophages. Mice expressing KIM-1Δmucin had greater functional impairment, inflammatory responses, and mortality in response to ischemia- and cisplatin-induced AKI. Compared with primary renal proximal tubule cells isolated from KIM-1Δmucin mice, those from WT mice had reduced proinflammatory cytokine secretion and impaired macrophage activation. The antiinflammatory effect of KIM-1 expression was due to the interaction of KIM-1 with p85 and subsequent PI3K-dependent downmodulation of NF-κB. Hence, KIM-1-mediated epithelial cell phagocytosis of apoptotic cells protects the kidney after acute injury by downregulating innate immunity and inflammation.
Figures








Comment in
-
Acute kidney injury: Proximal tubule cells modulate inflammation after renal injury.Nat Rev Nephrol. 2015 May;11(5):254. doi: 10.1038/nrneph.2015.40. Epub 2015 Mar 31. Nat Rev Nephrol. 2015. PMID: 25825087 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

