Acetyl-L-carnitine in the treatment of peripheral neuropathic pain: a systematic review and meta-analysis of randomized controlled trials
- PMID: 25751285
- PMCID: PMC4353712
- DOI: 10.1371/journal.pone.0119479
Acetyl-L-carnitine in the treatment of peripheral neuropathic pain: a systematic review and meta-analysis of randomized controlled trials
Erratum in
-
Correction: Acetyl-L-carnitine in the Treatment of Peripheral Neuropathic Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials.PLoS One. 2015 Jun 12;10(6):e0129991. doi: 10.1371/journal.pone.0129991. eCollection 2015. PLoS One. 2015. PMID: 26065423 Free PMC article. No abstract available.
-
Correction: Correction: Acetyl-L-carnitine in the Treatment of Peripheral Neuropathic Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials.PLoS One. 2015 Sep 23;10(9):e0139292. doi: 10.1371/journal.pone.0139292. eCollection 2015. PLoS One. 2015. PMID: 26398892 Free PMC article. No abstract available.
Abstract
Objective: Acetyl-L-carnitine (ALC), a constructive molecule in fatty acid metabolism, is an agent potentially effective for treating peripheral neuropathic pain (PNP). Its effect, however, remains uncertain. We aimed to access the efficacy and safety of ALC for the treatment of patients with PNP.
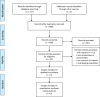
Methods: We searched MEDLINE (1996-2014), EMBase (1974-2014), and CENTRAL (May 2014) up to June 27, 2014 for randomized controlled trials (RCTs) comparing ALC with placebo or other active medications in diabetic and non-diabetic PNP patients that reported the change of pain using visual analogue scale (VAS). Mean difference (MD) and 95% confidence interval (CI) were used for pooling continuous data.
Results: Four RCTs comparing ALC with placebo and reporting in three articles (n = 523) were included. Compared with placebo, ALC significantly reduced VAS scores of PNP patients (MD of VAS, 1.20; 95% CI, 0.68-1.72, P <0.00001). In the subgroup analysis, the effect of ALC on VAS was similar in different administration routes (intramuscular-oral sequential subgroup: MD, 1.19; 95% CI, 0.34-2.04, P = 0.006; oral only subgroup: pooled MD, 1.15; 95%CI, 0.33-1.96, P = 0.006), and ALC appeared more effective in diabetic PNP patients than non-diabetic PNP patients (diabetic subgroup: MD, 1.47; 95%CI, 1.06-1.87, P <0.00001; non-diabetic subgroup: MD, 0.71; 95% CI, -0.01-1.43, P = 0.05). No severe adverse events were reported related to ALC. The common adverse events were pain, headache, paraesthesia, hyperesthesia, retching, biliary colic, and gastrointestinal disorders. The rates of total adverse events were similar in ALC and control group.
Conclusion: The current evidence suggests that ALC has a moderate effect in reducing pain measured on VAS in PNP patients with acceptable safety. Larger trials with longer follow-up, however, are warranted to establish the effects.
Conflict of interest statement
Figures
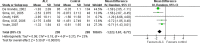


References
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

