An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells
- PMID: 25751305
- PMCID: PMC4404757
- DOI: 10.1038/nnano.2015.19
An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells
Abstract
In humans and other mammals it is known that calcium and phosphate ions are secreted from the distal small intestine into the lumen. However, why this secretion occurs is unclear. Here, we show that the process leads to the formation of amorphous magnesium-substituted calcium phosphate nanoparticles that trap soluble macromolecules, such as bacterial peptidoglycan and orally fed protein antigens, in the lumen and transport them to immune cells of the intestinal tissue. The macromolecule-containing nanoparticles utilize epithelial M cells to enter Peyer's patches, small areas of the intestine concentrated with particle-scavenging immune cells. In wild-type mice, intestinal immune cells containing these naturally formed nanoparticles expressed the immune tolerance-associated molecule 'programmed death-ligand 1', whereas in NOD1/2 double knockout mice, which cannot recognize peptidoglycan, programmed death-ligand 1 was undetected. Our results explain a role for constitutively formed calcium phosphate nanoparticles in the gut lumen and show how this helps to shape intestinal immune homeostasis.
Figures

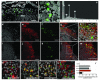



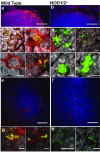
Comment in
-
Gut immunology: Nanoparticles ferry gut antigens.Nat Nanotechnol. 2015 Apr;10(4):298-9. doi: 10.1038/nnano.2015.58. Nat Nanotechnol. 2015. PMID: 25855255 No abstract available.
References
-
- Gomez-Morilla I, Thoree V, Powell JJ, Kirkby KJ, Grime GW. Identification and quantitative analysis of calcium phosphate microparticles in intestinal tissue by nuclear microscopy. Nucl. Instrum. Methods. Phys. Res. B. 2006;249:665–669.
-
- Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010;34:J226–233. - PubMed
-
- Geigy Scientific Tables. 8th edn Vol. 1. CIBA-GEIGY Limited; Basle, Switzerland: 1981.
-
- Schedl HP, Osbaldiston GW, Mills IH. Absorption, secretion, and precipitation of calcium in the small intestine of the dog. Am J Physiol. 1968;214:814–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

