Anti-incretin, Anti-proliferative Action of Dopamine on β-Cells
- PMID: 25751312
- PMCID: PMC4399277
- DOI: 10.1210/me.2014-1273
Anti-incretin, Anti-proliferative Action of Dopamine on β-Cells
Abstract
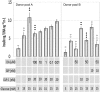
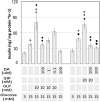
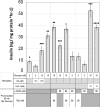
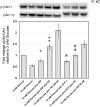
Human islet β-cells exploit an autocrine dopamine (DA)-mediated inhibitory circuit to regulate insulin secretion. β-Cells also express the DA active transporter and the large neutral amino acid transporter heterodimer enabling them to import circulating DA or its biosynthetic precursor, L-3,4-dihydroxyphenylalanine (L-DOPA). The capacity to import DA or L-DOPA from the extracellular space possibly indicates that DA may be an endocrine signal as well. In humans, a mixed meal stimulus is accompanied by contemporary serum excursions of incretins, DA and L-DOPA, suggesting that DA may act as an anti-incretin as postulated by the foregut hypothesis proposed to explain the early effects of bariatric surgery on type 2 diabetes. In this report, we take a translational step backwards and characterize the kinetics of plasma DA and incretin production after a mixed meal challenge in a rat model and study the integration of incretin and DA signaling at the biochemical level in a rodent β-cell line and islets. We found that there are similar excursions of incretins and DA in rats, as those reported in humans, after a mixed meal challenge and that DA counters incretin enhanced glucose-stimulated insulin secretion and intracellular signaling at multiple points from dampening calcium fluxes to inhibiting proliferation as well as apoptosis. Our data suggest that DA is an important regulator of insulin secretion and may represent 1 axis of a gut level circuit of glucose and β-cell mass homeostasis.
Figures




Similar articles
-
A role for foregut tyrosine metabolism in glucose tolerance.Mol Metab. 2019 May;23:37-50. doi: 10.1016/j.molmet.2019.02.008. Epub 2019 Feb 27. Mol Metab. 2019. PMID: 30876866 Free PMC article.
-
Pleiotropic effects of GIP on islet function involve osteopontin.Diabetes. 2011 Sep;60(9):2424-33. doi: 10.2337/db10-1532. Epub 2011 Aug 1. Diabetes. 2011. PMID: 21810601 Free PMC article.
-
Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus.J Clin Endocrinol Metab. 2003 Jun;88(6):2706-13. doi: 10.1210/jc.2002-021873. J Clin Endocrinol Metab. 2003. PMID: 12788877
-
Diabetes and obesity treatment based on dual incretin receptor activation: 'twincretins'.Diabetes Obes Metab. 2016 Sep;18(9):847-54. doi: 10.1111/dom.12685. Epub 2016 Jun 7. Diabetes Obes Metab. 2016. PMID: 27160961 Review.
-
Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and β cell preservation.Prog Biophys Mol Biol. 2011 Nov;107(2):248-56. doi: 10.1016/j.pbiomolbio.2011.07.010. Epub 2011 Jul 28. Prog Biophys Mol Biol. 2011. PMID: 21820006 Review.
Cited by
-
Gastrointestinal dopamine as an anti-incretin and its possible role in bypass surgery as therapy for type 2 diabetes with associated obesity.Minerva Endocrinol. 2016 Mar;41(1):43-56. Epub 2015 Oct 27. Minerva Endocrinol. 2016. PMID: 26505694 Free PMC article. Review.
-
A Novel and Selective Dopamine Transporter Inhibitor, (S)-MK-26, Promotes Hippocampal Synaptic Plasticity and Restores Effort-Related Motivational Dysfunctions.Biomolecules. 2022 Jun 24;12(7):881. doi: 10.3390/biom12070881. Biomolecules. 2022. PMID: 35883437 Free PMC article.
-
Dual pancreatic adrenergic and dopaminergic signaling as a therapeutic target of bromocriptine.iScience. 2022 Jul 19;25(8):104771. doi: 10.1016/j.isci.2022.104771. eCollection 2022 Aug 19. iScience. 2022. PMID: 35982797 Free PMC article.
-
Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes.Biomedicines. 2023 Nov 7;11(11):2993. doi: 10.3390/biomedicines11112993. Biomedicines. 2023. PMID: 38001993 Free PMC article. Review.
-
Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models.Nutrients. 2023 Mar 4;15(5):1281. doi: 10.3390/nu15051281. Nutrients. 2023. PMID: 36904281 Free PMC article.
References
-
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. - PubMed
-
- Pories WJ, MacDonald KG, Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55(2 suppl):582S–585S. - PubMed
-
- Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55(7):2025–2031. - PubMed
-
- Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252(6):966–971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

