NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking
- PMID: 25751534
- PMCID: PMC4376619
- DOI: 10.1038/nn.3972
NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking
Abstract
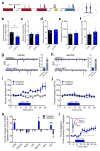
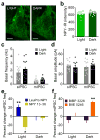
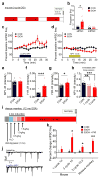
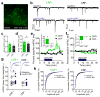
Binge alcohol drinking is a tremendous public health problem because it leads to the development of numerous pathologies, including alcohol abuse and anxiety. It is thought to do so by hijacking brain systems that regulate stress and reward, including neuropeptide Y (NPY) and corticotropin-releasing factor (CRF). The central actions of NPY and CRF have opposing functions in the regulation of emotional and reward-seeking behaviors; thus, dysfunctional interactions between these peptidergic systems could be involved in the development of these pathologies. We used converging physiological, pharmacological and chemogenetic approaches to identify a precise neural mechanism in the bed nucleus of the stria terminalis (BNST), a limbic brain region involved in pathological reward and anxiety behaviors, underlying the interactions between NPY and CRF in the regulation of binge alcohol drinking in both mice and monkeys. We found that NPY Y1 receptor (Y1R) activation in the BNST suppressed binge alcohol drinking by enhancing inhibitory synaptic transmission specifically in CRF neurons via a previously unknown Gi-mediated, PKA-dependent postsynaptic mechanism. Furthermore, chronic alcohol drinking led to persistent alterations in Y1R function in the BNST of both mice and monkeys, highlighting the enduring, conserved nature of this effect across mammalian species. Together, these data provide both a cellular locus and signaling framework for the development of new therapeutics for treatment of neuropsychiatric diseases, including alcohol use disorders.
Figures
Comment in
-
Neuroscience: Binge drinking and brain stress systems.Nature. 2015 Apr 9;520(7546):168-9. doi: 10.1038/520168a. Nature. 2015. PMID: 25855451 No abstract available.
Similar articles
-
Neuroscience: Binge drinking and brain stress systems.Nature. 2015 Apr 9;520(7546):168-9. doi: 10.1038/520168a. Nature. 2015. PMID: 25855451 No abstract available.
-
Chronic stress alters neuropeptide Y signaling in the bed nucleus of the stria terminalis in DBA/2J but not C57BL/6J mice.Neuropharmacology. 2012 Mar;62(4):1777-86. doi: 10.1016/j.neuropharm.2011.12.002. Epub 2011 Dec 9. Neuropharmacology. 2012. PMID: 22182779 Free PMC article.
-
Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis.Neuropharmacology. 2006 Oct;51(5):1013-22. doi: 10.1016/j.neuropharm.2006.06.011. Epub 2006 Aug 10. Neuropharmacology. 2006. PMID: 16904135
-
CRF modulation of central monoaminergic function: Implications for sex differences in alcohol drinking and anxiety.Alcohol. 2018 Nov;72:33-47. doi: 10.1016/j.alcohol.2018.01.007. Epub 2018 Feb 2. Alcohol. 2018. PMID: 30217435 Free PMC article. Review.
-
Interactions between NPY and CRF in the amygdala to regulate emotionality.Neuropeptides. 2004 Aug;38(4):225-34. doi: 10.1016/j.npep.2004.05.006. Neuropeptides. 2004. PMID: 15337374 Review.
Cited by
-
Effects of chronic alcohol consumption on neuronal function in the non-human primate BNST.Addict Biol. 2016 Nov;21(6):1151-1167. doi: 10.1111/adb.12289. Epub 2015 Jul 29. Addict Biol. 2016. PMID: 26223349 Free PMC article.
-
Corticotropin Releasing Factor Type 1 and 2 Receptor Signaling in the Medial Prefrontal Cortex Modulates Binge-Like Ethanol Consumption in C57BL/6J Mice.Brain Sci. 2019 Jul 19;9(7):171. doi: 10.3390/brainsci9070171. Brain Sci. 2019. PMID: 31330967 Free PMC article.
-
Mad men, women and steroid cocktails: a review of the impact of sex and other factors on anabolic androgenic steroids effects on affective behaviors.Psychopharmacology (Berl). 2016 Feb;233(4):549-69. doi: 10.1007/s00213-015-4193-6. Epub 2016 Jan 12. Psychopharmacology (Berl). 2016. PMID: 26758282 Free PMC article. Review.
-
The Periaqueductal Gray and Its Extended Participation in Drug Addiction Phenomena.Neurosci Bull. 2021 Oct;37(10):1493-1509. doi: 10.1007/s12264-021-00756-y. Epub 2021 Jul 24. Neurosci Bull. 2021. PMID: 34302618 Free PMC article. Review.
-
Commonalities and Distinctions Among Mechanisms of Addiction to Alcohol and Other Drugs.Alcohol Clin Exp Res. 2015 Oct;39(10):1863-77. doi: 10.1111/acer.12810. Alcohol Clin Exp Res. 2015. PMID: 26431116 Free PMC article. Review.
References
-
- Ilveskoski E, et al. Association of neuropeptide y polymorphism with the occurrence of type 1 and type 2 alcoholism. Alcohol Clin Exp Res. 2001;25:1420–1422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AA109431/AA/NIAAA NIH HHS/United States
- AA020911/AA/NIAAA NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- K08 DK071561/DK/NIDDK NIH HHS/United States
- AA013541/AA/NIAAA NIH HHS/United States
- AA013573/AA/NIAAA NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- AA019454/AA/NIAAA NIH HHS/United States
- R01 DK096010/DK/NIDDK NIH HHS/United States
- AA022048/AA/NIAAA NIH HHS/United States
- T32 GM008322/GM/NIGMS NIH HHS/United States
- R01 DK075632/DK/NIDDK NIH HHS/United States
- R01 DK089044/DK/NIDDK NIH HHS/United States
- AA011605/AA/NIAAA NIH HHS/United States
- F32 AA021043/AA/NIAAA NIH HHS/United States
- AA021043/AA/NIAAA NIH HHS/United States
- DK057521/DK/NIDDK NIH HHS/United States
- DK075632/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- DK046200/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- R01 AA019454/AA/NIAAA NIH HHS/United States
- P50 AA011605/AA/NIAAA NIH HHS/United States
- T32 DA007244/DA/NIDA NIH HHS/United States
- U01 AA020911/AA/NIAAA NIH HHS/United States
- R37 AA013573/AA/NIAAA NIH HHS/United States
- DK071561/DK/NIDDK NIH HHS/United States
- DK096010/DK/NIDDK NIH HHS/United States
- R01 AA013573/AA/NIAAA NIH HHS/United States
- P60 AA011605/AA/NIAAA NIH HHS/United States
- R01 AA022048/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

