Ceramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phoshate
- PMID: 25751724
- PMCID: PMC4394466
- DOI: 10.3390/ijms16035076
Ceramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phoshate
Abstract
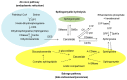
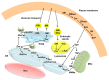
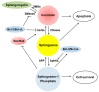
Ceramide is synthesized upon stimuli, and induces apoptosis in renal tubular cells (RTCs). Sphingosine-1 phosphate (S1P) functions as a survival factor. Thus, the balance of ceramide/S1P determines ceramide-induced apoptosis. Mitochondria play a key role for ceramide-induced apoptosis by altered mitochondrial outer membrane permeability (MOMP). Ceramide enhances oligomerization of pro-apoptotic Bcl-2 family proteins, ceramide channel, and reduces anti-apoptotic Bcl-2 proteins in the MOM. This process alters MOMP, resulting in generation of reactive oxygen species (ROS), cytochrome C release into the cytosol, caspase activation, and apoptosis. Ceramide regulates apoptosis through mitogen-activated protein kinases (MAPKs)-dependent and -independent pathways. Conversely, MAPKs alter ceramide generation by regulating the enzymes involving ceramide metabolism, affecting ceramide-induced apoptosis. Crosstalk between Bcl-2 family proteins, ROS, and many signaling pathways regulates ceramide-induced apoptosis. Growth factors rescue ceramide-induced apoptosis by regulating the enzymes involving ceramide metabolism, S1P, and signaling pathways including MAPKs. This article reviews evidence supporting a role of ceramide for apoptosis and discusses a role of mitochondria, including MOMP, Bcl-2 family proteins, ROS, and signaling pathways, and crosstalk between these factors in the regulation of ceramide-induced apoptosis of RTCs. A balancing role between ceramide and S1P and the strategy for preventing ceramide-induced apoptosis by growth factors are also discussed.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

