The mesosphere and metals: chemistry and changes
- PMID: 25751779
- PMCID: PMC4448204
- DOI: 10.1021/cr500501m
The mesosphere and metals: chemistry and changes
Figures

























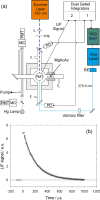










References
-
- Plane J. M. C. Int. Rev. Phys. Chem. 1991, 10, 55.
-
- Plane J. M. C. Chem. Rev. 2003, 103, 4963. - PubMed
-
- Plane J. M. C.; Helmer M. In Research in Chemical Kinetics; Compton R. G., Hancock G., Eds.; Elsevier Science: Amsterdam, 1994.
-
- Plane J. M. C. In Meteors in the Earth’s Atmosphere; Murad E., Williams I. P., Eds.; Cambridge University Press: Cambridge, U.K., 2002.
-
- McNeil W. J.; Murad E.; Plane J. M. C. In Meteors in the Earth’s Atmosphere; Murad E., Williams I. P., Eds.; Cambridge University Press: Cambridge, U.K., 2002.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

