Assessing chemical constituents of Mimosa caesalpiniifolia stem bark: possible bioactive components accountable for the cytotoxic effect of M. caesalpiniifolia on human tumour cell lines
- PMID: 25751783
- PMCID: PMC6272184
- DOI: 10.3390/molecules20034204
Assessing chemical constituents of Mimosa caesalpiniifolia stem bark: possible bioactive components accountable for the cytotoxic effect of M. caesalpiniifolia on human tumour cell lines
Abstract
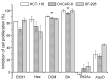
Mimosa caesalpiniifolia is a native plant of the Brazilian northeast, and few studies have investigated its chemical composition and biological significance. This work describes the identification of the first chemical constituents in the ethanolic extract and fractions of M. caesalpiniifolia stem bark based on NMR, GC-qMS and HRMS analyses, as well as an assessment of their cytotoxic activity. GC-qMS analysis showed fatty acid derivatives, triterpenes and steroid substances and confirmed the identity of the chemical compounds isolated from the hexane fraction. Metabolite biodiversity in M. caesalpiniifolia stem bark revealed the differentiated accumulation of pentacyclic triterpenic acids, with a high content of betulinic acid and minor amounts of 3-oxo and 3β-acetoxy derivatives. Bioactive analysis based on total phenolic and flavonoid content showed a high amount of these compounds in the ethanolic extract, and ESI-(-)-LTQ-Orbitrap-MS identified caffeoyl hexose at high intensity, as well as the presence of phenolic acids and flavonoids. Furthermore, the evaluation of the ethanolic extract and fractions, including betulinic acid, against colon (HCT-116), ovarian (OVCAR-8) and glioblastoma (SF-295) tumour cell lines showed that the crude extract, hexane and dichloromethane fractions possessed moderate to high inhibitory activity, which may be related to the abundance of betulinic acid. The phytochemical and biological study of M. caesalpiniifolia stem bark thus revealed a new alternative source of antitumour compounds, possibly made effective by the presence of betulinic acid and by chemical co-synergism with other compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dutra V.F., Garcia F.C.P. Three new species of Mimosa sect. Mimosa (Leguminosae, Mimosoideae) from the campos rupestres of Minas Gerais, Brazil. Brittonia. 2014;66:33–41.
-
- Martel-Estrada S.A., Olivas-Armendáriz I., Santos-Rodríguez E., Martínez-Pérez C.A., García-Casillas P.E., Hernández-Paz J., Rodríguez-González C.A., Chapa-González C. Evaluation of in vitro bioactivity of chitosan/Mimosa tenuiflora composites. Mater. Lett. 2014;119:146–149. doi: 10.1016/j.matlet.2014.01.004. - DOI
-
- Aguiar R.M., Alves C.Q., David J.M., Rezende L.C., Lima L.S., David J.P., Queiróz L.P. Antioxidant activities of isolated compounds from stems of Mimosa invisa Mart. ex. Colla. Quim. Nova. 2012;35:567–570. doi: 10.1590/S0100-40422012000300024. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

