Stem cell treatment of degenerative eye disease
- PMID: 25752437
- PMCID: PMC4434205
- DOI: 10.1016/j.scr.2015.02.003
Stem cell treatment of degenerative eye disease
Abstract
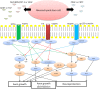
Stem cell therapies are being explored extensively as treatments for degenerative eye disease, either for replacing lost neurons, restoring neural circuits or, based on more recent evidence, as paracrine-mediated therapies in which stem cell-derived trophic factors protect compromised endogenous retinal neurons from death and induce the growth of new connections. Retinal progenitor phenotypes induced from embryonic stem cells/induced pluripotent stem cells (ESCs/iPSCs) and endogenous retinal stem cells may replace lost photoreceptors and retinal pigment epithelial (RPE) cells and restore vision in the diseased eye, whereas treatment of injured retinal ganglion cells (RGCs) has so far been reliant on mesenchymal stem cells (MSC). Here, we review the properties of non-retinal-derived adult stem cells, in particular neural stem cells (NSCs), MSC derived from bone marrow (BMSC), adipose tissues (ADSC) and dental pulp (DPSC), together with ESC/iPSC and discuss and compare their potential advantages as therapies designed to provide trophic support, repair and replacement of retinal neurons, RPE and glia in degenerative retinal diseases. We conclude that ESCs/iPSCs have the potential to replace lost retinal cells, whereas MSC may be a useful source of paracrine factors that protect RGC and stimulate regeneration of their axons in the optic nerve in degenerate eye disease. NSC may have potential as both a source of replacement cells and also as mediators of paracrine treatment.
Copyright © 2015. Published by Elsevier B.V.
Figures

References
-
- Aanismaa R., Hautala J., Vuorinen A., Miettinen S., Narkilahti S. Human dental pulp stem cells differentiate into neural precursors but not into mature functional neurons. Stem Cell Discov. 2012;2:85–91.
-
- Ahmad I., Tang L., Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 2000;270:517–521. - PubMed
-
- Anghileri E., Marconi S., Pignatelli A., Cifelli P., Galie M., Sbarbati A., Krampera M., Belluzzi O., Bonetti B. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:909–916. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

