Apoptosis Signal-regulating Kinase 1 (ASK1)-p38 Pathway-dependent Cytoplasmic Translocation of the Orphan Nuclear Receptor NR4A2 Is Required for Oxidative Stress-induced Necrosis
- PMID: 25752609
- PMCID: PMC4409244
- DOI: 10.1074/jbc.M114.623280
Apoptosis Signal-regulating Kinase 1 (ASK1)-p38 Pathway-dependent Cytoplasmic Translocation of the Orphan Nuclear Receptor NR4A2 Is Required for Oxidative Stress-induced Necrosis
Abstract
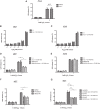
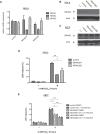
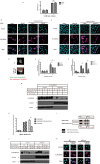
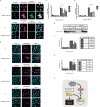
p38 mitogen-activated protein kinases (MAPKs) play important roles in various cellular stress responses, including cell death, which is roughly categorized into apoptosis and necrosis. Although p38 signaling has been extensively studied, the molecular mechanisms of p38-mediated cell death are unclear. ASK1 is a stress-responsive MAP3K that acts as an upstream kinase of p38 and is activated by various stresses, such as oxidative stress. Here, we show that NR4A2, a member of the NR4A nuclear receptor family, acts as a necrosis promoter downstream of ASK1-p38 pathway during oxidative stress. Although NR4A2 is well known as a nucleus-localized transcription factor, we found that it is translocated into the cytosol after phosphorylation by p38. Because the phosphorylation site mutants of NR4A2 cannot rescue the cell death-promoting activity, ASK1-p38 pathway-dependent phosphorylation and subsequent cytoplasmic translocation of NR4A2 may be required for oxidative stress-induced cell death. In addition, NR4A2-mediated cell death does not depend on caspases and receptor-interacting protein 1 (RIP1)-RIP3 complex, suggesting that NR4A2 promotes an RIP kinase-independent necrotic type of cell death. Our findings may enable a more precise understanding of molecular mechanisms that regulate oxidative stress-induced and p38-mediated necrosis.
Keywords: Necrosis; Nuclear Receptor; Oxidative Stress; Phosphorylation; Signal Transduction; Stress Response; p38 MAPK.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90–94 - PubMed
-
- Nagai H., Noguchi T., Homma K., Katagiri K., Takeda K., Matsuzawa A., Ichijo H. (2009) Ubiquitin-like sequence in ASK1 plays critical roles in the recognition and stabilization by USP9X and oxidative stress-induced cell death. Mol. Cell 36, 805–818 - PubMed
-
- Noguchi T., Ishii K., Fukutomi H., Naguro I., Matsuzawa A., Takeda K., Ichijo H. (2008) Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J. Biol. Chem. 283, 7657–7665 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

