UreE-UreG complex facilitates nickel transfer and preactivates GTPase of UreG in Helicobacter pylori
- PMID: 25752610
- PMCID: PMC4432268
- DOI: 10.1074/jbc.M114.632364
UreE-UreG complex facilitates nickel transfer and preactivates GTPase of UreG in Helicobacter pylori
Abstract
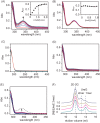
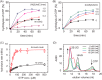
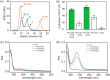
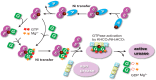
The pathogenicity of Helicobacter pylori relies heavily on urease, which converts urea to ammonia to neutralize the stomach acid. Incorporation of Ni(2+) into the active site of urease requires a battery of chaperones. Both metallochaperones UreE and UreG play important roles in the urease activation. In this study, we demonstrate that, in the presence of GTP and Mg(2+), UreG binds Ni(2+) with an affinity (Kd) of ∼0.36 μm. The GTPase activity of Ni(2+)-UreG is stimulated by both K(+) (or NH4 (+)) and HCO3 (-) to a biologically relevant level, suggesting that K(+)/NH4 (+) and HCO3 (-) might serve as GTPase elements of UreG. We show that complexation of UreE and UreG results in two protein complexes, i.e. 2E-2G and 2E-G, with the former being formed only in the presence of both GTP and Mg(2+). Mutagenesis studies reveal that Arg-101 on UreE and Cys-66 on UreG are critical for stabilization of 2E-2G complex. Combined biophysical and bioassay studies show that the formation of 2E-2G complex not only facilitates nickel transfer from UreE to UreG, but also enhances the binding of GTP. This suggests that UreE might also serve as a structural scaffold for recruitment of GTP to UreG. Importantly, we demonstrate for the first time that UreE serves as a bridge to grasp Ni(2+) from HypA, subsequently donating it to UreG. The study expands our horizons on the molecular details of nickel translocation among metallochaperones UreE, UreG, and HypA, which further extends our knowledge on the urease maturation process.
Keywords: Helicobacter pylori; Metal Ion-Protein Interaction; Metallochaperone; Metalloprotein; Nickel; Protein-Protein Interaction; Translocation; UreE; UreG; Urease; Urease Maturation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Structural insights into how GTP-dependent conformational changes in a metallochaperone UreG facilitate urease maturation.Proc Natl Acad Sci U S A. 2017 Dec 19;114(51):E10890-E10898. doi: 10.1073/pnas.1712658114. Epub 2017 Dec 4. Proc Natl Acad Sci U S A. 2017. PMID: 29203664 Free PMC article.
-
Helicobacter pylori hydrogenase accessory protein HypA and urease accessory protein UreG compete with each other for UreE recognition.Biochim Biophys Acta. 2012 Oct;1820(10):1519-25. doi: 10.1016/j.bbagen.2012.06.002. Epub 2012 Jun 12. Biochim Biophys Acta. 2012. PMID: 22698670 Free PMC article.
-
The Helicobacter pylori HypA·UreE2 Complex Contains a Novel High-Affinity Ni(II)-Binding Site.Biochemistry. 2018 May 22;57(20):2932-2942. doi: 10.1021/acs.biochem.8b00127. Epub 2018 May 10. Biochemistry. 2018. PMID: 29708738 Free PMC article.
-
Moving nickel along the hydrogenase-urease maturation pathway.Metallomics. 2022 May 13;14(5):mfac003. doi: 10.1093/mtomcs/mfac003. Metallomics. 2022. PMID: 35556134 Review.
-
Biosynthesis of the urease metallocenter.J Biol Chem. 2013 May 10;288(19):13178-85. doi: 10.1074/jbc.R112.446526. Epub 2013 Mar 28. J Biol Chem. 2013. PMID: 23539618 Free PMC article. Review.
Cited by
-
The requirement for cobalt in vitamin B12: A paradigm for protein metalation.Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118896. doi: 10.1016/j.bbamcr.2020.118896. Epub 2020 Oct 21. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33096143 Free PMC article. Review.
-
Targeting the Protein Tunnels of the Urease Accessory Complex: A Theoretical Investigation.Molecules. 2020 Jun 24;25(12):2911. doi: 10.3390/molecules25122911. Molecules. 2020. PMID: 32599898 Free PMC article.
-
Mechanistic Insights into the Metal-Dependent Activation of ZnII-Dependent Metallochaperones.Inorg Chem. 2019 Oct 21;58(20):13661-13672. doi: 10.1021/acs.inorgchem.9b01173. Epub 2019 Jun 17. Inorg Chem. 2019. PMID: 31247880 Free PMC article.
-
Non-thiolate ligation of nickel by nucleotide-free UreG of Klebsiella aerogenes.J Biol Inorg Chem. 2017 Jun;22(4):497-503. doi: 10.1007/s00775-016-1429-9. Epub 2016 Dec 21. J Biol Inorg Chem. 2017. PMID: 28004186 Free PMC article.
-
A metal-trap tests and refines blueprints to engineer cellular protein metalation with different elements.Nat Commun. 2025 Jan 18;16(1):810. doi: 10.1038/s41467-025-56199-w. Nat Commun. 2025. PMID: 39827241 Free PMC article.
References
-
- Warren J. R., Marshall B. (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 321, 1273–1275 - PubMed
-
- Covacci A., Telford J. L., Del Giudice G., Parsonnet J., Rappuoli R. (1999) Helicobacter pylori virulence and genetic geography. Science 284, 1328–1333 - PubMed
-
- Maier R. J., Fu C., Gilbert J., Moshiri F., Olson J., Plaut A. G. (1996) Hydrogen uptake hydrogenase in Helicobacter pylori. FEMS Microbiol. Lett. 141, 71–76 - PubMed
-
- Evans D. J., Jr., Evans D. G., Kirkpatrick S. S., Graham D. Y. (1991) Characterization of the Helicobacter pylori urease and purification of its subunits. Microb. Pathog. 10, 15–26 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

