High structural resolution hydroxyl radical protein footprinting reveals an extended Robo1-heparin binding interface
- PMID: 25752613
- PMCID: PMC4409239
- DOI: 10.1074/jbc.M115.648410
High structural resolution hydroxyl radical protein footprinting reveals an extended Robo1-heparin binding interface
Abstract
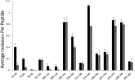
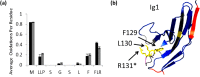
Interaction of transmembrane receptors of the Robo family and the secreted protein Slit provides important signals in the development of the central nervous system and regulation of axonal midline crossing. Heparan sulfate, a sulfated linear polysaccharide modified in a complex variety of ways, serves as an essential co-receptor in Slit-Robo signaling. Previous studies have shown that closely related heparin octasaccharides bind to Drosophila Robo directly, and surface plasmon resonance analysis revealed that Robo1 binds more tightly to full-length unfractionated heparin. For the first time, we utilized electron transfer dissociation-based high spatial resolution hydroxyl radical protein footprinting to identify two separate binding sites for heparin interaction with Robo1: one binding site at the previously identified site for heparin dp8 and a second binding site at the N terminus of Robo1 that is disordered in the x-ray crystal structure. Mutagenesis of the identified N-terminal binding site exhibited a decrease in binding affinity as measured by surface plasmon resonance and heparin affinity chromatography. Footprinting also indicated that heparin binding induces a minor change in the conformation and/or dynamics of the Ig2 domain, but no major conformational changes were detected. These results indicate a second low affinity binding site in the Robo-Slit complex as well as suggesting the role of the Ig2 domain of Robo1 in heparin-mediated signal transduction. This study also marks the first use of electron transfer dissociation-based high spatial resolution hydroxyl radical protein footprinting, which shows great utility for the characterization of protein-carbohydrate complexes.
Keywords: Glycosaminoglycan; Heparin; Heparin-binding Protein; Hydroxyl Radical Protein Footprinting; Mass Spectrometry (MS); Protein-Carbohydrate Interaction; Structural Biology.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



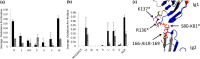


References
-
- Garbe D. S., Bashaw G. J. (2004) Axon guidance at the midline: from mutants to mechanisms. Crit. Rev. Biochem. Mol. Biol. 39, 319–341 - PubMed
-
- Dickson B. J., Gilestro G. F. (2006) Regulation of commissural axon pathfinding by slit and its robo receptors. Annu. Rev. Cell Dev. Biol. 22, 651–675 - PubMed
-
- Kidd T., Brose K., Mitchell K. J., Fetter R. D., Tessier-Lavigne M., Goodman C. S., Tear G. (1998) Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 92, 205–215 - PubMed
-
- Huminiecki L., Gorn M., Suchting S., Poulsom R., Bicknell R. (2002) Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics 79, 547–552 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

