Multiple forms of metaplasticity at a single hippocampal synapse during late postnatal development
- PMID: 25752732
- PMCID: PMC4887277
- DOI: 10.1016/j.dcn.2015.01.009
Multiple forms of metaplasticity at a single hippocampal synapse during late postnatal development
Erratum in
-
Corrigendum to "Multiple forms of metaplasticity at a single hippocampal synapse during late postnatal development" [Dev. Cogn. Sci. 12C (2015) 145-154].Dev Cogn Neurosci. 2016 Aug;20:1. doi: 10.1016/j.dcn.2016.05.006. Epub 2016 Jun 6. Dev Cogn Neurosci. 2016. PMID: 27495364 Free PMC article. No abstract available.
Abstract
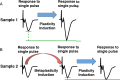
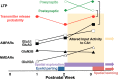
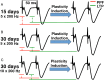
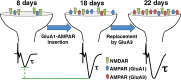
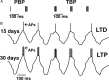
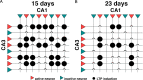
Metaplasticity refers to adjustment in the requirements for induction of synaptic plasticity based on the prior history of activity. Numerous forms of developmental metaplasticity are observed at Schaffer collateral synapses in the rat hippocampus at the end of the third postnatal week. Emergence of spatial learning and memory at this developmental stage suggests possible involvement of metaplasticity in the final maturation of the hippocampus. Three distinct metaplastic phenomena are apparent. (1) As transmitter release probability increases with increasing age, presynaptic potentiation is reduced. (2) Alterations in the composition and channel conductance properties of AMPARs facilitate the induction of postsynaptic potentiation with increasing age. (3) Low frequency stimulation inhibits subsequent induction of potentiation in animals older but not younger than 3 weeks of age. Thus, many forms of plasticity expressed at SC-CA1 synapses are different in rats younger and older than 3 weeks of age, illustrating the complex orchestration of physiological modifications that underlie the maturation of hippocampal excitatory synaptic transmission. This review paper describes three late postnatal modifications to synaptic plasticity induction in the hippocampus and attempts to relate these metaplastic changes to developmental alterations in hippocampal network activity and the maturation of contextual learning.
Keywords: Hippocampus; Long-term depression; Long-term potentiation; Metaplasticity; Postnatal development; Schaffer collateral.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Abraham W.C., Bear M.F. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Bekenstein J.W., Lothman E.W. A comparison of the ontogeny of excitatory and inhibitory neurotransmission in the CA1 region and dentate gyrus of the rat hippocampal formation. Dev. Brain Res. 1991;63:237–243. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

