Human papillomavirus molecular biology and disease association
- PMID: 25752814
- PMCID: PMC5024016
- DOI: 10.1002/rmv.1822
Human papillomavirus molecular biology and disease association
Abstract
Human papillomaviruses (HPVs) have evolved over millions of years to propagate themselves in a range of different animal species including humans. Viruses that have co-evolved slowly in this way typically cause chronic inapparent infections, with virion production in the absence of apparent disease. This is the case for many Beta and Gamma HPV types. The Alpha papillomavirus types have however evolved immunoevasion strategies that allow them to cause persistent visible papillomas. These viruses activate the cell cycle as the infected epithelial cell differentiates in order to create a replication competent environment that allows viral genome amplification and packaging into infectious particles. This is mediated by the viral E6, E7, and E5 proteins. High-risk E6 and E7 proteins differ from their low-risk counterparts however in being able to drive cell cycle entry in the upper epithelial layers and also to stimulate cell proliferation in the basal and parabasal layers. Deregulated expression of these cell cycle regulators underlies neoplasia and the eventual progression to cancer in individuals who cannot resolve high-risk HPV infection. Most work to date has focused on the study of high-risk HPV types such as HPV 16 and 18, which has led to an understanding of the molecular pathways subverted by these viruses. Such approaches will lead to the development of better strategies for disease treatment, including targeted antivirals and immunotherapeutics. Priorities are now focused toward understanding HPV neoplasias at sites other than the cervix (e.g. tonsils, other transformation zones) and toward understanding the mechanisms by which low-risk HPV types can sometimes give rise to papillomatosis and under certain situations even cancers.
Copyright © 2015 John Wiley & Sons, Ltd.
Figures
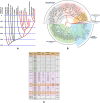


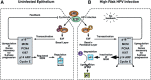

References
-
- Bravo IG, de Sanjose S, Gottschling M. The clinical importance of understanding the evolution of papillomaviruses. Trends in Microbiology 2010; 18(10): 432–438. - PubMed
-
- Antonsson A, McMillan NA. Papillomavirus in healthy skin of Australian animals. Journal of General Virology 2006; 87(Pt 11): 3195–3200. - PubMed
-
- Griffiths P. Time to consider the concept of a commensal virus? Reviews in Medical Virology 1999; 9(2): 73–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

