From gross anatomy to the nanomorphome: stereological tools provide a paradigm for advancing research in quantitative morphomics
- PMID: 25753334
- PMCID: PMC4386931
- DOI: 10.1111/joa.12287
From gross anatomy to the nanomorphome: stereological tools provide a paradigm for advancing research in quantitative morphomics
Abstract
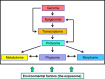
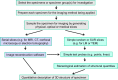
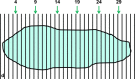
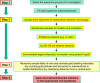
The terms morphome and morphomics are not new but, recently, a group of morphologists and cell biologists has given them clear definitions and emphasised their integral importance in systems biology. By analogy to other '-omes', the morphome refers to the distribution of matter within 3-dimensional (3D) space. It equates to the totality of morphological features within a biological system (virus, single cell, multicellular organism or populations thereof) and morphomics is the systematic study of those structures. Morphomics research has the potential to generate 'big data' because it includes all imaging techniques at all levels of achievable resolution and all structural scales from gross anatomy and medical imaging, via optical and electron microscopy, to molecular characterisation. As with other '-omics', quantification is an important part of morphomics and, because biological systems exist and operate in 3D space, precise descriptions of form, content and spatial relationships require the quantification of structure in 3D. Revealing and quantifying structural detail inside the specimen is achieved currently in two main ways: (i) by some form of reconstruction from serial physical or tomographic slices or (ii) by using randomly-sampled sections and simple test probes (points, lines, areas, volumes) to derive stereological estimates of global and/or individual quantities. The latter include volumes, surfaces, lengths and numbers of interesting features and spatial relationships between them. This article emphasises the value of stereological design, sampling principles and estimation tools as a template for combining with alternative imaging techniques to tackle the 'big data' issue and advance knowledge and understanding of the morphome. The combination of stereology, TEM and immunogold cytochemistry provides a practical illustration of how this has been achieved in the sub-field of nanomorphomics. Applying these quantitative tools/techniques in a carefully managed study design offers us a deeper appreciation of the spatiotemporal relationships between the genome, metabolome and morphome which are integral to systems biology.
Keywords: electron microscopy; immunogold cytochemistry; morphome; morphomics; quantifying 3D structure; stereology.
© 2015 Anatomical Society.
Figures

References
-
- Amiry-Moghaddam M, Ottersen OP. Immunogold cytochemistry in neuroscience. Nat Neurosci. 2013;7:798–804. - PubMed
-
- Aoyama K, Takagi T, Hirase A, et al. STEM tomography for thick biological specimens. Ultramicroscopy. 2008;109:70–80. - PubMed
-
- Baddeley AJ, Gundersen HJG, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. - PubMed
-
- Blazquez-Llorca L, Merchán-Pérez A, Rodríguez JR, et al. FIB/SEM technology and Alzheimer's Disease: three-dimensional analysis of human cortical synapses. J Alzheimers Dis. 2013;34:995–1013. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

