The interaction of anticancer therapies with tumor-associated macrophages
- PMID: 25753580
- PMCID: PMC4387285
- DOI: 10.1084/jem.20150295
The interaction of anticancer therapies with tumor-associated macrophages
Abstract
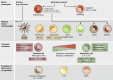
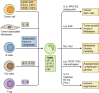
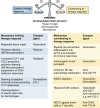
Macrophages are essential components of the inflammatory microenvironment of tumors. Conventional treatment modalities (chemotherapy and radiotherapy), targeted drugs, antiangiogenic agents, and immunotherapy, including checkpoint blockade, all profoundly influence or depend on the function of tumor-associated macrophages (TAMs). Chemotherapy and radiotherapy can have dual influences on TAMs in that a misdirected macrophage-orchestrated tissue repair response can result in chemoresistance, but in other circumstances, TAMs are essential for effective therapy. A better understanding of the interaction of anticancer therapies with innate immunity, and TAMs in particular, may pave the way to better patient selection and innovative combinations of conventional approaches with immunotherapy.
© 2015 Mantovani and Allavena.
Figures
References
-
- Affara N.I., Ruffell B., Medler T.R., Gunderson A.J., Johansson M., Bornstein S., Bergsland E., Steinhoff M., Li Y., Gong Q., et al. . 2014. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 25:809–821 10.1016/j.ccr.2014.04.026 - DOI - PMC - PubMed
-
- Aharinejad S., Paulus P., Sioud M., Hofmann M., Zins K., Schäfer R., Stanley E.R., and Abraham D.. 2004. Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res. 64:5378–5384 10.1158/0008-5472.CAN-04-0961 - DOI - PubMed
-
- Alizadeh D., Trad M., Hanke N.T., Larmonier C.B., Janikashvili N., Bonnotte B., Katsanis E., and Larmonier N.. 2014. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 74:104–118 10.1158/0008-5472.CAN-13-1545 - DOI - PMC - PubMed
-
- Bain C.C., Bravo-Blas A., Scott C.L., Gomez Perdiguero E., Geissmann F., Henri S., Malissen B., Osborne L.C., Artis D., and Mowat A.M.. 2014. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 15:929–937 10.1038/ni.2967 - DOI - PMC - PubMed
-
- Beatty G.L., Chiorean E.G., Fishman M.P., Saboury B., Teitelbaum U.R., Sun W., Huhn R.D., Song W., Li D., Sharp L.L., et al. . 2011. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 331:1612–1616 10.1126/science.1198443 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

