Fungal β-1,3-glucan increases ofloxacin tolerance of Escherichia coli in a polymicrobial E. coli/Candida albicans biofilm
- PMID: 25753645
- PMCID: PMC4432160
- DOI: 10.1128/AAC.04650-14
Fungal β-1,3-glucan increases ofloxacin tolerance of Escherichia coli in a polymicrobial E. coli/Candida albicans biofilm
Abstract
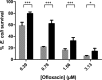
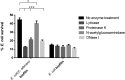
In the past, biofilm-related research has focused mainly on axenic biofilms. However, in nature, biofilms are often composed of multiple species, and the resulting polymicrobial interactions influence industrially and clinically relevant outcomes such as performance and drug resistance. In this study, we show that Escherichia coli does not affect Candida albicans tolerance to amphotericin or caspofungin in an E. coli/C. albicans biofilm. In contrast, ofloxacin tolerance of E. coli is significantly increased in a polymicrobial E. coli/C. albicans biofilm compared to its tolerance in an axenic E. coli biofilm. The increased ofloxacin tolerance of E. coli is mainly biofilm specific, as ofloxacin tolerance of E. coli is less pronounced in polymicrobial E. coli/C. albicans planktonic cultures. Moreover, we found that ofloxacin tolerance of E. coli decreased significantly when E. coli/C. albicans biofilms were treated with matrix-degrading enzymes such as the β-1,3-glucan-degrading enzyme lyticase. In line with a role for β-1,3-glucan in mediating ofloxacin tolerance of E. coli in a biofilm, we found that ofloxacin tolerance of E. coli increased even more in E. coli/C. albicans biofilms consisting of a high-β-1,3-glucan-producing C. albicans mutant. In addition, exogenous addition of laminarin, a polysaccharide composed mainly of poly-β-1,3-glucan, to an E. coli biofilm also resulted in increased ofloxacin tolerance. All these data indicate that β-1,3-glucan from C. albicans increases ofloxacin tolerance of E. coli in an E. coli/C. albicans biofilm.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Calderon K, Gonzalez-Martinez A, Gomez-Silvan C, Osorio F, Rodelas B, Gonzalez-Lopez J. 2013. Archaeal diversity in biofilm technologies applied to treat urban and industrial wastewater: recent advances and future prospects. Int J Mol Sci 14:18572–18598. doi:10.3390/ijms140918572. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

