Physical characterization and in vitro biological impact of highly aggregated antibodies separated into size-enriched populations by fluorescence-activated cell sorting
- PMID: 25753756
- PMCID: PMC4448733
- DOI: 10.1002/jps.24379
Physical characterization and in vitro biological impact of highly aggregated antibodies separated into size-enriched populations by fluorescence-activated cell sorting
Abstract
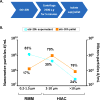
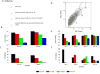
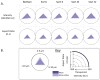
An IgG2 monoclonal antibody (mAb) solution was subjected to stirring, generating high concentrations of nanometer and subvisible particles, which were then successfully size-enriched into different size bins by low-speed centrifugation or a combination of gravitational sedimentation and fluorescence-activated cell sorting (FACS). The size-fractionated mAb particles were assessed for their ability to elicit the release of cytokines from a population of donor-derived human peripheral blood mononuclear cells (PBMC) at two phases of the immune response. Fractions enriched in nanometer-sized particles showed a lower response than those enriched in micron-sized particles in this assay. Particles of 5-10 μm in size displayed elevated cytokine release profiles compared with other size ranges. Stir-stressed mAb particles had amorphous morphology, contained protein with partially altered secondary structure, elevated surface hydrophobicity (compared with controls), and trace levels of elemental fluorine. FACS size-enriched the mAb particle samples, yet did not notably alter the overall morphology or composition of particles as measured by microflow imaging, transmission electron microscopy, and scanning electron microscopy-energy dispersive X-ray spectroscopy. The utility and limitations of FACS for size separation of mAb particles and potential of in vitro PBMC studies to rank-order the immunogenic potential of various types of mAb particles are discussed.
Keywords: IgG; immune response; immunogenicity; PBMC; in vitro; monoclonal antibody; particles; protein aggregation; proteins.
© 2015 Wiley Periodicals, Inc. and the American Pharmacists Association.
Figures






References
-
- Boven K, Knight J, Bader F, Rossert J, Eckardt KU, Casadevall N. Epoetin-associated pure red cell aplasia in patients with chronic kidney disease: solving the mystery. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20(Suppl 3):iii33–40. - PubMed
-
- Casadevall N. What is antibody-mediated pure red cell aplasia (PRCA)? Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20(Suppl 4):iv3–8. - PubMed
-
- Chirino AJ, Ary ML, Marshall SA. Minimizing the immunogenicity of protein therapeutics. Drug Discov Today. 2004;9(2):82–90. - PubMed
-
- Claman HN. Tolerance to a Protein Antigen in Adult Mice and the Effect of Nonspecific Factors. Journal of Immunology. 1963;91:833–839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

