A QM-MD simulation approach to the analysis of FRET processes in (bio)molecular systems. A case study: complexes of E. coli purine nucleoside phosphorylase and its mutants with formycin A
- PMID: 25754135
- PMCID: PMC4353879
- DOI: 10.1007/s00894-015-2602-8
A QM-MD simulation approach to the analysis of FRET processes in (bio)molecular systems. A case study: complexes of E. coli purine nucleoside phosphorylase and its mutants with formycin A
Abstract
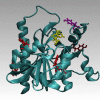
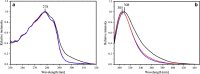
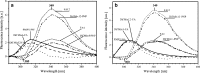
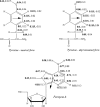
Predicting FRET pathways in proteins using computer simulation techniques is very important for reliable interpretation of experimental data. A novel and relatively simple methodology has been developed and applied to purine nucleoside phosphorylase (PNP) complexed with a fluorescent ligand - formycin A (FA). FRET occurs between an excited Tyr residue (D*) and FA (A). This study aims to interpret experimental data that, among others, suggests the absence of FRET for the PNPF159A mutant in complex with FA, based on novel theoretical methodology. MD simulations for the protein molecule containing D*, and complexed with A, are carried out. Interactions of D* with its molecular environment are accounted by including changes of the ESP charges in S1, compared to S0, and computed at the SCF-CI level. FRET probability W F depends on the inverse six-power of the D*-A distance, R da . The orientational factor 0 < k(2) < 4 between D* and A is computed and included in the analysis. Finally W F is time-averaged over the MD trajectories resulting in its mean value. The red-shift of the tyrosinate anion emission and thus lack of spectral overlap integral and thermal energy dissipation are the reasons for the FRET absence in the studied mutants at pH 7 and above. The presence of the tyrosinate anion results in a competitive energy dissipation channel and red-shifted emission, thus in consequence in the absence of FRET. These studies also indicate an important role of the phenyl ring of Phe159 for FRET in the wild-type PNP, which does not exist in the Ala159 mutant, and for the effective association of PNP with FA. In a more general context, our observations point out very interesting and biologically important properties of the tyrosine residue in its excited state, which may undergo spontaneous deprotonation in the biomolecular systems, resulting further in unexpected physical and/or biological phenomena. Until now, this observation has not been widely discussed in the literature.
Figures









Similar articles
-
Towards understanding the E. coli PNP binding mechanism and FRET absence between E. coli PNP and formycin A.Biophys Chem. 2017 Nov;230:99-108. doi: 10.1016/j.bpc.2017.09.001. Epub 2017 Sep 19. Biophys Chem. 2017. PMID: 28947300
-
Formycin A and its N-methyl analogues, specific inhibitors of E. coli purine nucleoside phosphorylase (PNP): induced tautomeric shifts on binding to enzyme, and enzyme-->ligand fluorescence resonance energy transfer.Biochim Biophys Acta. 2000 Jan 3;1476(1):109-28. doi: 10.1016/s0167-4838(99)00225-3. Biochim Biophys Acta. 2000. PMID: 10606773
-
A synergistic effect of phosphate, pH and Phe159 substitution on the formycin A association to the E. coli purine nucleoside phosphorylase.Biochimie. 2018 May;148:80-86. doi: 10.1016/j.biochi.2018.02.012. Epub 2018 Feb 28. Biochimie. 2018. PMID: 29499297
-
A new approach to interpretation of heterogeneity of fluorescence decay: effect of induced tautomeric shift and enzyme-->ligand fluorescence resonance energy transfer.Biophys Chem. 2006 Sep 20;123(2-3):146-53. doi: 10.1016/j.bpc.2006.05.014. Epub 2006 Jun 12. Biophys Chem. 2006. PMID: 16765509
-
PNP anticancer gene therapy.Curr Top Med Chem. 2005;5(13):1259-74. doi: 10.2174/156802605774463105. Curr Top Med Chem. 2005. PMID: 16305530 Review.
References
-
- Kierdaszuk B, Modrak-Wójcik A, Wierzchowski J, Shugar D. Formycin A and its N-methyl analogues, specific inhibitor of E. coli Purine nucleoside phosphorylase (PNP): induced tautomeric shift on binding to enzyme, and enzyme-ligand fluorescence resonance energy transfer. Biochim Biophys Acta. 2000;1476:109–128. doi: 10.1016/S0167-4838(99)00225-3. - DOI - PubMed
-
- Hirshfield MS, Chaffe S, Koro-Johnson L, Mary A, Smith AA, Short SA. Use of site-directed mutagenesis to enhance the epitope-shielding effect of covalent modification of proteins with polyethylene glycol. Proc Natl Acad Sci U S A. 1991;88:7185–7189. doi: 10.1073/pnas.88.16.7185. - DOI - PMC - PubMed
-
- Kalckar HM. The enzymatic synthesis of purine-ribosides. J Biol Chem. 1947;167:429–443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

