PG545 enhances anti-cancer activity of chemotherapy in ovarian models and increases surrogate biomarkers such as VEGF in preclinical and clinical plasma samples
- PMID: 25754234
- PMCID: PMC4402130
- DOI: 10.1016/j.ejca.2015.02.007
PG545 enhances anti-cancer activity of chemotherapy in ovarian models and increases surrogate biomarkers such as VEGF in preclinical and clinical plasma samples
Abstract
Background: Despite the utility of antiangiogenic drugs in ovarian cancer, efficacy remains limited due to resistance linked to alternate angiogenic pathways and metastasis. Therefore, we investigated PG545, an anti-angiogenic and anti-metastatic agent which is currently in Phase I clinical trials, using preclinical models of ovarian cancer.
Methods: PG545's anti-cancer activity was investigated in vitro and in vivo as a single agent, and in combination with paclitaxel, cisplatin or carboplatin using various ovarian cancer cell lines and tumour models.
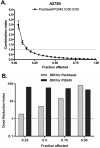
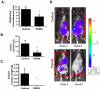
Results: PG545, alone, or in combination with chemotherapeutics, inhibited proliferation of ovarian cancer cells, demonstrating synergy with paclitaxel in A2780 cells. PG545 inhibited growth factor-mediated cell migration and reduced HB-EGF-induced phosphorylation of ERK, AKT and EGFR in vitro and significantly reduced tumour burden which was enhanced when combined with paclitaxel in an A2780 model or carboplatin in a SKOV-3 model. Moreover, in the immunocompetent ID8 model, PG545 also significantly reduced ascites in vivo. In the A2780 maintenance model, PG545 initiated with, and following paclitaxel and cisplatin treatment, significantly improved overall survival. PG545 increased plasma VEGF levels (and other targets) in preclinical models and in a small cohort of advanced cancer patients which might represent a potential biomarker of response.
Conclusion: Our results support clinical testing of PG545, particularly in combination with paclitaxel, as a novel therapeutic strategy for ovarian cancer.
Keywords: HB-EGF; Heparanase; Ovarian cancer; PG545; Tumour microenvironment; VEGF.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





References
-
- Davidson B, Shafat I, Risberg B, Ilan N, Trope’ CG, Vlodavsky I, et al. Heparanase expression correlates with poor survival in metastatic ovarian carcinoma. Gynecol Oncol. 2007;104:311–9. doi:10.1016/j.ygyno.2006.08.045. - PubMed
-
- Zheng H, Ruan J, Zhao P, Chen S, Pan L, Liu J. Heparanase is involved in proliferation and invasion of ovarian cancer cells. Cancer Biomark. 2015 doi:10.3233/CBM-150459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

