The Spemann organizer meets the anterior-most neuroectoderm at the equator of early gastrulae in amphibian species
- PMID: 25754292
- PMCID: PMC4402005
- DOI: 10.1111/dgd.12200
The Spemann organizer meets the anterior-most neuroectoderm at the equator of early gastrulae in amphibian species
Abstract
The dorsal blastopore lip (known as the Spemann organizer) is important for making the body plan in amphibian gastrulation. The organizer is believed to involute inward and migrate animally to make physical contact with the prospective head neuroectoderm at the blastocoel roof of mid- to late-gastrula. However, we found that this physical contact was already established at the equatorial region of very early gastrula in a wide variety of amphibian species. Here we propose a unified model of amphibian gastrulation movement. In the model, the organizer is present at the blastocoel roof of blastulae, moves vegetally to locate at the region that lies from the blastocoel floor to the dorsal lip at the onset of gastrulation. The organizer located at the blastocoel floor contributes to the anterior axial mesoderm including the prechordal plate, and the organizer at the dorsal lip ends up as the posterior axial mesoderm. During the early step of gastrulation, the anterior organizer moves to establish the physical contact with the prospective neuroectoderm through the "subduction and zippering" movements. Subduction makes a trench between the anterior organizer and the prospective neuroectoderm, and the tissues face each other via the trench. Zippering movement, with forming Brachet's cleft, gradually closes the gap to establish the contact between them. The contact is completed at the equator of early gastrulae and it continues throughout the gastrulation. After the contact is established, the dorsal axis is formed posteriorly, but not anteriorly. The model also implies the possibility of constructing a common model of gastrulation among chordate species.
Keywords: Spemann organizer; amphibian; chordate; gastrulation; movement.
© 2015 The Authors Development, Growth & Differentiation published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Society of Developmental Biologists.
Figures
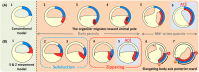
 , organizer;
, organizer;  , prospective neuroectoderm.
, prospective neuroectoderm.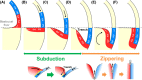
 , organizer;
, organizer;  , prospective neuroectoderm.
, prospective neuroectoderm.
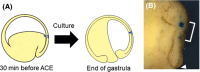
 , Bombina orientalis;
, Bombina orientalis;  , Rana japonica;
, Rana japonica;  , Rana porosa brevipoda;
, Rana porosa brevipoda;  , Rana rugosa;
, Rana rugosa;  , Silurana tropicalis;
, Silurana tropicalis;  , Xenopus laevis;
, Xenopus laevis;  , Ambystoma mexicanum;
, Ambystoma mexicanum;  , Cynops ensicauda;
, Cynops ensicauda;  , Cynops pyrrhogaster;
, Cynops pyrrhogaster;  , Hynobius nebulosus.
, Hynobius nebulosus.





References
-
- Blitz IL. Cho KW. Anterior neuroectoderm is progressively induced during gastrulation the role of the Xenopus homeobox gene orthodenticle. Development. 1995;121:993–1004. - PubMed
-
- Boucaut JC, Darribere T, Boulekbache H. Thiery JP. Prevention of gastrulation but not neurulation by antibodies to fibronectin in amphibian embryos. Nature. 1984;307:364–367. - PubMed
-
- Bouwmeester T, Kim SH, Sasai Y, Lu B. De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. - PubMed
-
- Butler K, Zorn AM. Gurdon JB. Non radioactive in situ hybridization to Xenopus tissue sections. Methods. 2001;23:303–312. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

