Heparanase released from mesenchymal stem cells activates integrin beta1/HIF-2alpha/Flk-1 signaling and promotes endothelial cell migration and angiogenesis
- PMID: 25754303
- PMCID: PMC5108061
- DOI: 10.1002/stem.1995
Heparanase released from mesenchymal stem cells activates integrin beta1/HIF-2alpha/Flk-1 signaling and promotes endothelial cell migration and angiogenesis
Erratum in
-
Corrigendum.Stem Cells. 2019 May;37(5):E3. doi: 10.1002/stem.3019. Stem Cells. 2019. PMID: 31034132 No abstract available.
Abstract
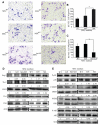
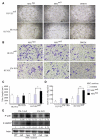
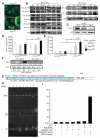
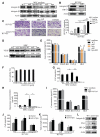
Heparanase plays important roles in tumor angiogenesis. Our previous study demonstrated that hypoxic preconditioning (HPC) enhanced the angiogenic and therapeutic effects of mesenchymal stem cells (MSCs), effects that were paralleled by enhanced heparanase expression. This study was designed to elucidate the role of heparanase in the improved therapeutic properties of HPC-MSCs and to explore underlying mechanisms using an ischemic rat hind limb model. MSCs transfected with heparanase (MSC(hpa) ) or empty vector (MSC(null) ) were delivered by intramuscular injections to ischemic hind limbs. Hind limbs that received MSC(hpa) recovered blood flow more rapidly at 7 days and acquired higher capillary density at 14 days compared with MSC(null) . Conditioned medium from MSC(hpa) increased endothelial cell migration and promoted greater tube formation relative to that from the MSC(null) groups. Vascular endothelial growth factor receptor 2 (VEGFR2, Flk-1) and its downstream signaling pathway (p38MAPK/HSP27) were significantly increased in human umbilical vein endothelial cells (HUVECs) after treatment with MSC(hpa) conditioned medium. Each of these responses was decreased by cocultured with MSC(hpa-KD) conditioned medium. MSC(hpa) conditioned medium activated hypoxia-inducible factor-2α (HIF-2α) and increased in parallel the transcript level of Flk-1 as determined by chromatin immunoprecipitation-PCR and luciferase assays. Analyses of integrin expression revealed an important role for integrin β1 in the regulation of HIF-2α. All angiogenic effects of MSC(hpa) conditioned medium were abolished by knockdown of integrin β1, HIF-2α, and Flk-1 in HUVECs with selective shRNAs. These findings identify heparanse as a key regulator of angiogenesis by MSCs. We propose a novel pathway wherein heparanse sequentially activates integrin β1, HIF-2α, Flk-1, and p38MAPK/HSP27 with corresponding enhancement of angiogenesis.
Keywords: Angiogenesis; Heparanase; Mesenchymal stem cell.
© 2015 AlphaMed Press.
Figures



References
-
- Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007;8:35–42. - PubMed
-
- Poitevin S, Cussac D, Leroyer AS, et al. Sphingosine kinase 1 expressed by endothelial colony-forming cells has a critical role in their revascularization activity. Cardiovasc Res. 2014;103:121–130. - PubMed
-
- Chamberlain G, Fox J, Ashton B, et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

