Ligand-enabled meta-C-H activation using a transient mediator
- PMID: 25754328
- PMCID: PMC4368492
- DOI: 10.1038/nature14214
Ligand-enabled meta-C-H activation using a transient mediator
Abstract
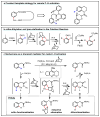
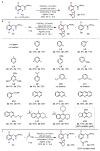
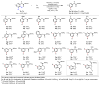
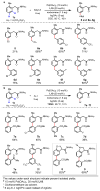
Achieving site selectivity in C-H functionalization reactions is a significant challenge, especially when the target C-H bond is distant from existing functional groups. Coordination of a functional group to a metal is often a key driving force and control element in many important reactions including asymmetric hydrogenation, epoxidation and lithiation. Exploitation of this effect has led to the development of a broad range of directed C-H activation reactions. However, these C-H activation methods are limited to proximal C-H bonds, which are spatially and geometrically accessible from the directing functional group. The development of meta-selective C-H functionalizations remains a significant challenge. We recently developed a U-shaped template that can be used to overcome this constraint and have shown that it can be used to selectively activate remote meta-C-H bonds. Although this approach has proved to be applicable to various substrates and catalytic transformations, the need for a covalently attached, complex template is a substantial drawback for synthetic applications. Here we report an alternative approach employing norbornene as a transient mediator to achieve meta-selective C-H activation with a simple and common ortho-directing group. The use of a newly developed pyridine-based ligand is crucial for relaying the palladium catalyst to the meta position by norbornene after initial ortho-C-H activation. This catalytic reaction demonstrates the feasibility of switching ortho-selectivity to meta-selectivity in C-H activation of the same substrate by catalyst control.
Conflict of interest statement
The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article.
Figures
References
-
- Yang G, et al. Pd(II)-catalyzed meta-C–H olefination, arylation, and acetoxylation of indolines using a U-shaped template. J Am Chem Soc. 2014;136:10807–10813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

