Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development
- PMID: 25754609
- PMCID: PMC4366535
- DOI: 10.1038/ncomms7356
Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development
Abstract
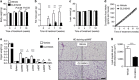
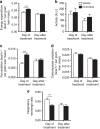
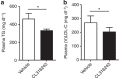
Brown adipose tissue (BAT) combusts high amounts of fatty acids, thereby lowering plasma triglyceride levels and reducing obesity. However, the precise role of BAT in plasma cholesterol metabolism and atherosclerosis development remains unclear. Here we show that BAT activation by β3-adrenergic receptor stimulation protects from atherosclerosis in hyperlipidemic APOE*3-Leiden.CETP mice, a well-established model for human-like lipoprotein metabolism that unlike hyperlipidemic Apoe(-/-) and Ldlr(-/-) mice expresses functional apoE and LDLR. BAT activation increases energy expenditure and decreases plasma triglyceride and cholesterol levels. Mechanistically, we demonstrate that BAT activation enhances the selective uptake of fatty acids from triglyceride-rich lipoproteins into BAT, subsequently accelerating the hepatic clearance of the cholesterol-enriched remnants. These effects depend on a functional hepatic apoE-LDLR clearance pathway as BAT activation in Apoe(-/-) and Ldlr(-/-) mice does not attenuate hypercholesterolaemia and atherosclerosis. We conclude that activation of BAT is a powerful therapeutic avenue to ameliorate hyperlipidaemia and protect from atherosclerosis.
Figures






Comment in
-
Dyslipidaemia: Active BAT reduces atherosclerosis.Nat Rev Endocrinol. 2015 Jun;11(6):317. doi: 10.1038/nrendo.2015.50. Epub 2015 Mar 31. Nat Rev Endocrinol. 2015. PMID: 25824681 No abstract available.
References
-
- Jukema J. W., Cannon C. P., de Craen A. J., Westendorp R. G. & Trompet S. The controversies of statin therapy: weighing the evidence. J. Am. Coll. Cardiol. 60, 875–881 (2012) . - PubMed
-
- Hany T. F. et al. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur. J. Nucl. Med. Mol. Imaging 29, 1393–1398 (2002) . - PubMed
-
- Nedergaard J., Bengtsson T. & Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 (2007) . - PubMed
-
- van Marken Lichtenbelt W. D. et al. Cold-activated brown adipose tissue in healthy men. New Engl. J. Med. 360, 1500–1508 (2009) . - PubMed
-
- Virtanen K. A. et al. Functional brown adipose tissue in healthy adults. New Engl. J. Med. 360, 1518–1525 (2009) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

