Myosin 18A coassembles with nonmuscle myosin 2 to form mixed bipolar filaments
- PMID: 25754640
- PMCID: PMC8760901
- DOI: 10.1016/j.cub.2015.02.012
Myosin 18A coassembles with nonmuscle myosin 2 to form mixed bipolar filaments
Abstract
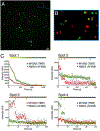
Class-18 myosins are most closely related to conventional class-2 nonmuscle myosins (NM2). Surprisingly, the purified head domains of Drosophila, mouse, and human myosin 18A (M18A) lack actin-activated ATPase activity and the ability to translocate actin filaments, suggesting that the functions of M18A in vivo do not depend on intrinsic motor activity. M18A has the longest coiled coil of any myosin outside of the class-2 myosins, suggesting that it might form bipolar filaments similar to conventional myosins. To address this possibility, we expressed and purified full-length mouse M18A using the baculovirus/Sf9 system. M18A did not form large bipolar filaments under any of the conditions tested. Instead, M18A formed an ∼ 65-nm-long bipolar structure with two heads at each end. Importantly, when NM2 was polymerized in the presence of M18A, the two myosins formed mixed bipolar filaments, as evidenced by cosedimentation, electron microscopy, and single-molecule imaging. Moreover, super-resolution imaging of NM2 and M18A using fluorescently tagged proteins and immunostaining of endogenous proteins showed that NM2 and M18A are present together within individual filaments inside living cells. Together, our in vitro and live-cell imaging data argue strongly that M18A coassembles with NM2 into mixed bipolar filaments. M18A could regulate the biophysical properties of these filaments and, by virtue of its extra N- and C-terminal domains, determine the localization and/or molecular interactions of the filaments. Given the numerous, fundamental cellular and developmental roles attributed to NM2, our results have far-reaching biological implications.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
-
- Lowey S, Slayter HS, Weeds AG, and Baker H (1969). Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol 42, 1–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

