Sleep interacts with aβ to modulate intrinsic neuronal excitability
- PMID: 25754641
- PMCID: PMC4366315
- DOI: 10.1016/j.cub.2015.01.016
Sleep interacts with aβ to modulate intrinsic neuronal excitability
Abstract
Background: Emerging data suggest an important relationship between sleep and Alzheimer's disease (AD), but how poor sleep promotes the development of AD remains unclear.
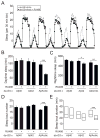
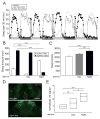
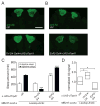
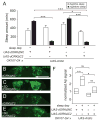
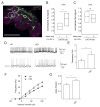
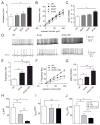
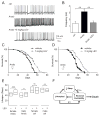
Results: Here, using a Drosophila model of AD, we provide evidence suggesting that changes in neuronal excitability underlie the effects of sleep loss on AD pathogenesis. β-amyloid (Aβ) accumulation leads to reduced and fragmented sleep, while chronic sleep deprivation increases Aβ burden. Moreover, enhancing sleep reduces Aβ deposition. Increasing neuronal excitability phenocopies the effects of reducing sleep on Aβ, and decreasing neuronal activity blocks the elevated Aβ accumulation induced by sleep deprivation. At the single neuron level, we find that chronic sleep deprivation, as well as Aβ expression, enhances intrinsic neuronal excitability. Importantly, these data reveal that sleep loss exacerbates Aβ-induced hyperexcitability and suggest that defects in specific K(+) currents underlie the hyperexcitability caused by sleep loss and Aβ expression. Finally, we show that feeding levetiracetam, an anti-epileptic medication, to Aβ-expressing flies suppresses neuronal excitability and significantly prolongs their lifespan.
Conclusions: Our findings directly link sleep loss to changes in neuronal excitability and Aβ accumulation and further suggest that neuronal hyperexcitability is an important mediator of Aβ toxicity. Taken together, these data provide a mechanistic framework for a positive feedback loop, whereby sleep loss and neuronal excitation accelerate the accumulation of Aβ, a key pathogenic step in the development of AD.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
-
- Moe KE, Vitiello MV, Larsen LH, Prinz PN. Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res. 1995;4:15–20. - PubMed
-
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:361–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

