Candida-streptococcal mucosal biofilms display distinct structural and virulence characteristics depending on growth conditions and hyphal morphotypes
- PMID: 25754666
- PMCID: PMC4491045
- DOI: 10.1111/omi.12095
Candida-streptococcal mucosal biofilms display distinct structural and virulence characteristics depending on growth conditions and hyphal morphotypes
Abstract
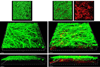
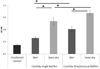
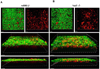
Candida albicans and streptococci of the mitis group form communities in multiple oral sites, where moisture and nutrient availability can change spatially or temporally. This study evaluated structural and virulence characteristics of Candida-streptococcal biofilms formed on moist or semidry mucosal surfaces, and tested the effects of nutrient availability and hyphal morphotype on dual-species biofilms. Three-dimensional models of the oral mucosa formed by immortalized keratinocytes on a fibroblast-embedded collagenous matrix were used. Infections were carried out using Streptococcus oralis strain 34, in combination with a C. albicans wild-type strain, or pseudohyphal-forming mutant strains. Increased moisture promoted a homogeneous surface biofilm by C. albicans. Dual biofilms had a stratified structure, with streptococci growing in close contact with the mucosa and fungi growing on the bacterial surface. Under semidry conditions, Candida formed localized foci of dense growth, which promoted focal growth of streptococci in mixed biofilms. Candida biofilm biovolume was greater under moist conditions, albeit with minimal tissue invasion, compared with semidry conditions. Supplementing the infection medium with nutrients under semidry conditions intensified growth, biofilm biovolume and tissue invasion/damage, without changing biofilm structure. Under these conditions, the pseudohyphal mutants and S. oralis formed defective superficial biofilms, with most bacteria in contact with the epithelial surface, below a pseudohyphal mass, resembling biofilms growing in a moist environment. The presence of S. oralis promoted fungal invasion and tissue damage under all conditions. We conclude that moisture, nutrient availability, hyphal morphotype and the presence of commensal bacteria influence the architecture and virulence characteristics of mucosal fungal biofilms.
Keywords: Candida; Streptococcus; biofilms; infection models.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

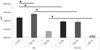








Similar articles
-
Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle.Mol Oral Microbiol. 2017 Feb;32(1):60-73. doi: 10.1111/omi.12154. Epub 2016 Mar 15. Mol Oral Microbiol. 2017. PMID: 26834007
-
S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms.Virulence. 2017 Nov 17;8(8):1602-1617. doi: 10.1080/21505594.2017.1326438. Epub 2017 Jun 1. Virulence. 2017. PMID: 28481721 Free PMC article.
-
Biofilm Interactions of Candida albicans and Mitis Group Streptococci in a Titanium-Mucosal Interface Model.Appl Environ Microbiol. 2020 Apr 17;86(9):e02950-19. doi: 10.1128/AEM.02950-19. Print 2020 Apr 17. Appl Environ Microbiol. 2020. PMID: 32111586 Free PMC article.
-
Germ tube growth of Candida albicans.Curr Top Med Mycol. 1997 Dec;8(1-2):43-55. Curr Top Med Mycol. 1997. PMID: 9504066 Review.
-
Pathogenic factors in Candida biofilm-related infectious diseases.J Appl Microbiol. 2017 Feb;122(2):321-330. doi: 10.1111/jam.13330. Epub 2016 Nov 24. J Appl Microbiol. 2017. PMID: 27770500 Review.
Cited by
-
The oral microbiota: dynamic communities and host interactions.Nat Rev Microbiol. 2018 Dec;16(12):745-759. doi: 10.1038/s41579-018-0089-x. Nat Rev Microbiol. 2018. PMID: 30301974 Free PMC article. Review.
-
Host's Immunity and Candida Species Associated with Denture Stomatitis: A Narrative Review.Microorganisms. 2022 Jul 16;10(7):1437. doi: 10.3390/microorganisms10071437. Microorganisms. 2022. PMID: 35889156 Free PMC article. Review.
-
Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model.Mol Oral Microbiol. 2018 Jun;33(3):212-223. doi: 10.1111/omi.12214. Epub 2018 Feb 20. Mol Oral Microbiol. 2018. PMID: 29314782 Free PMC article.
-
Role of Candida albicans secreted aspartyl protease Sap9 in interkingdom biofilm formation.Pathog Dis. 2016 Apr;74(3):ftw005. doi: 10.1093/femspd/ftw005. Epub 2016 Jan 14. Pathog Dis. 2016. PMID: 26772652 Free PMC article.
-
The role of salivary metabolomics in chronic periodontitis: bridging oral and systemic diseases.Metabolomics. 2025 Feb 7;21(1):24. doi: 10.1007/s11306-024-02220-0. Metabolomics. 2025. PMID: 39920480 Free PMC article. Review.
References
-
- Brown DH, Jr., Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 1999;34:651–662. - PubMed
-
- Cassone A, Cauda R. Candida and candidiasis in HIV-infected patients: where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS. 2012;26:1457–1472. - PubMed
-
- Cavalcanti YW, Morse DJ, da Silva WJ, Del-Bel-Cury AA, Wei X, Wilson M, Milward P, Lewis M, Bradshaw D, Williams DW. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling. 2015;31:27–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

