Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass
- PMID: 25755084
- PMCID: PMC4380834
- DOI: 10.1002/oby.21043
Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass
Abstract
Objective: Hyperinsulinemic hypoglycemia with neuroglycopenia is a rare complication following Roux-en-Y gastric bypass (RYGB) surgery for weight management. Insulin secretion and action in response to oral and intravenous stimuli in persons with and without neuroglycopenia following RYGB are evaluated in this study.
Methods: Cross-sectional cohort studies were performed at a single academic institution to assess insulin secretion and action during oral mixed meal tolerance test and intravenous glucose tolerance test (IVGTT).
Results: Insulin secretion was increased more following oral mixed meal than intravenous glucose in individuals with neuroglycopenia compared to the asymptomatic group. Reduced insulin clearance did not contribute to higher insulinemia. Glucose effectiveness at zero insulin, estimated during the IVGTT, was also higher in those with neuroglycopenia. Insulin sensitivity did not differ between groups.
Conclusions: Increased beta-cell response to oral stimuli and insulin-independent glucose disposal may both contribute to severe hypoglycemia after RYGB.
© 2015 The Obesity Society.
Conflict of interest statement
Figures
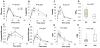



References
-
- Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. - PubMed
-
- Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. The New England Journal of Medicine. 2005;353:249–254. - PubMed
-
- Marsk R, Jonas E, Rasmussen F, Naslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia. 2010;53:2307–2311. - PubMed
-
- Sarwar H, Chapman WH, 3rd, Pender JR, Ivanescu A, Drake AJ, 3rd, Pories WJ, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obesity Surgery. 2014;24:1120–1124. - PubMed
-
- Kellogg TA, Bantle JP, Leslie DB, Redmond JB, Slusarek B, Swan T, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surgery for Obesity and Related Diseases. 2008;4:492–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

