Spectral imaging to measure heterogeneity in membrane lipid packing
- PMID: 25755090
- PMCID: PMC4539592
- DOI: 10.1002/cphc.201402794
Spectral imaging to measure heterogeneity in membrane lipid packing
Abstract
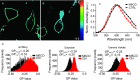
Physicochemical properties of the plasma membrane have been shown to play an important role in cellular functionality. Among those properties, the molecular order of the lipids, or the lipid packing, is of high importance. Changes in lipid packing are believed to compartmentalize cellular signaling by initiating coalescence and conformational changes of proteins. A common way to infer membrane lipid packing is by using membrane-embedded polarity-sensitive dyes, whose emission spectrum is dependent on the molecular order of the immediate membrane environment. Here, we report on an improved determination of such spectral shifts in the emission spectrum of the polarity-sensitive dyes. This improvement is based on the use of spectral imaging on a scanning confocal fluorescence microscope in combination with an improved analysis, which considers the whole emission spectrum instead of just single wavelength ranges. Using this approach and the polarity-sensitive dyes C-Laurdan or Di-4-ANEPPDHQ, we were able to image-with high accuracy-minute differences in the lipid packing of model and cellular membranes.
Keywords: C-laurdan; generalized polarization; lipid packing; membrane rafts; spectral imaging.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

