Community-based walking exercise for peripheral artery disease: An exploratory pilot study
- PMID: 25755148
- PMCID: PMC4749131
- DOI: 10.1177/1358863X15572725
Community-based walking exercise for peripheral artery disease: An exploratory pilot study
Abstract
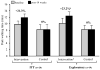
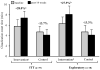
Supervised walking exercise is an effective treatment to improve walking ability of patients with peripheral artery disease (PAD), but few exercise programs in community settings have been effective. The aim of this study was to determine the efficacy of a community-based walking exercise program with training, monitoring and coaching (TMC) components to improve exercise performance and patient-reported outcomes in PAD patients. This was a randomized, controlled trial including PAD patients (n=25) who previously received peripheral endovascular therapy or presented with stable claudication. Patients randomized to the intervention group received a comprehensive community-based walking exercise program with elements of TMC over 14 weeks. Patients in the control group did not receive treatment beyond standard advice to walk. The primary outcome in the intent-to-treat (ITT) analyses was peak walking time (PWT) on a graded treadmill. Secondary outcomes included claudication onset time (COT) and patient-reported outcomes assessed via the Walking Impairment Questionnaire (WIQ). Intervention group patients (n=10) did not significantly improve PWT when compared with the control group patients (n=10) (mean ± standard error: +2.1 ± 0.7 versus 0.0 ± 0.7 min, p=0.052). Changes in COT and WIQ scores were greater for intervention patients compared with control patients (COT: +1.6 ± 0.8 versus -0.6 ± 0.7 min, p=0.045; WIQ: +18.3 ± 4.2 versus -4.6 ± 4.2%, p=0.001). This pilot using a walking program with TMC and an ITT analysis did not improve the primary outcome in PAD patients. Other walking performance and patient self-reported outcomes were improved following exercise in community settings. Further study is needed to determine whether this intervention improves outcomes in a trial employing a larger sample size.
Keywords: activity monitors; claudication; environmental audit; unsupervised exercise.
© The Author(s) 2015.
Conflict of interest statement
Dr. Hiatt is the director of a clinical trials research organization (CPC Clinical Research) that manages trials evaluating new treatments for PAD. Sponsors for relevant projects include Pluristem, AstraZeneca, Bayer, the Cardiovascular Cell Therapy Research Network and Cardiovascular Systems, Inc. The other authors note no conflicts.
Figures
References
-
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197–1206. - PubMed
-
- Creager MA, Libby P. Peripheral artery diseases. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald's heart disease: A textbook of cardiovascular medicine. 9. Philadelphia, PA: Elsevier; 2011. pp. 1338–1358.
-
- Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328–333. - PubMed
-
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Transatlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239–1312. - PubMed
-
- Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996;94(11):3026–3049. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

