Evaluating an etiologically relevant platform for therapy development for temporal lobe epilepsy: effects of carbamazepine and valproic acid on acute seizures and chronic behavioral comorbidities in the Theiler's murine encephalomyelitis virus mouse model
- PMID: 25755209
- PMCID: PMC4407718
- DOI: 10.1124/jpet.114.222513
Evaluating an etiologically relevant platform for therapy development for temporal lobe epilepsy: effects of carbamazepine and valproic acid on acute seizures and chronic behavioral comorbidities in the Theiler's murine encephalomyelitis virus mouse model
Abstract
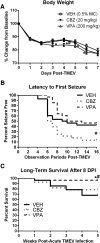
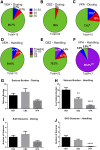
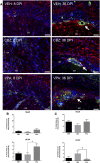
Central nervous system infections can underlie the development of epilepsy, and Theiler's murine encephalomyelitis virus (TMEV) infection in C57BL/6J mice provides a novel model of infection-induced epilepsy. Approximately 50-65% of infected mice develop acute, handling-induced seizures during the infection. Brains display acute neuropathology, and a high number of mice develop spontaneous, recurrent seizures and behavioral comorbidities weeks later. This study characterized the utility of this model for drug testing by assessing whether antiseizure drug treatment during the acute infection period attenuates handling-induced seizures, and whether such treatment modifies associated comorbidities. Male C57BL/6J mice infected with TMEV received twice-daily valproic acid (VPA; 200 mg/kg), carbamazepine (CBZ; 20 mg/kg), or vehicle during the infection (days 0-7). Mice were assessed twice daily during the infection period for handling-induced seizures. Relative to vehicle-treated mice, more CBZ-treated mice presented with acute seizures; VPA conferred no change. In mice displaying seizures, VPA, but not CBZ, reduced seizure burden. Animals were then randomly assigned to acute and long-term follow-up. VPA was associated with significant elevations in acute (day 8) glial fibrillary acidic protein (astrocytes) immunoreactivity, but did not affect NeuN (neurons) immunoreactivity. Additionally, VPA-treated mice showed improved motor performance 15 days postinfection (DPI). At 36 DPI, CBZ-treated mice traveled significantly less distance through the center of an open field, indicative of anxiety-like behavior. CBZ-treated mice also presented with significant astrogliosis 36 DPI. Neither CBZ nor VPA prevented long-term reductions in NeuN immunoreactivity. The TMEV model thus provides an etiologically relevant platform to evaluate potential treatments for acute seizures and disease modification.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Acute treatment with minocycline, but not valproic acid, improves long-term behavioral outcomes in the Theiler's virus model of temporal lobe epilepsy.Epilepsia. 2016 Dec;57(12):1958-1967. doi: 10.1111/epi.13577. Epub 2016 Oct 14. Epilepsia. 2016. PMID: 27739576 Free PMC article.
-
Intestinal dysbiosis alters acute seizure burden and antiseizure medicine activity in Theiler's virus model of encephalitis.Epilepsia. 2025 Aug;66(8):3022-3034. doi: 10.1111/epi.18395. Epub 2025 Mar 28. Epilepsia. 2025. PMID: 40153196
-
Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler's virus model of temporal lobe epilepsy.Neurobiol Dis. 2014 Apr;64:98-106. doi: 10.1016/j.nbd.2013.12.015. Epub 2014 Jan 9. Neurobiol Dis. 2014. PMID: 24412221 Free PMC article.
-
Facets of Theiler's Murine Encephalomyelitis Virus-Induced Diseases: An Update.Int J Mol Sci. 2019 Jan 21;20(2):448. doi: 10.3390/ijms20020448. Int J Mol Sci. 2019. PMID: 30669615 Free PMC article. Review.
-
Standard approach to antiepileptic drug treatment in the United States.Epilepsia. 1994;35 Suppl 4:S11-8. doi: 10.1111/j.1528-1157.1994.tb05951.x. Epilepsia. 1994. PMID: 8174515 Review.
Cited by
-
Diet composition and sterilization modifies intestinal microbiome diversity and burden of Theiler's virus infection-induced acute seizures.bioRxiv [Preprint]. 2023 Oct 17:2023.10.17.562694. doi: 10.1101/2023.10.17.562694. bioRxiv. 2023. Update in: Epilepsia. 2024 Jun;65(6):1777-1790. doi: 10.1111/epi.17946. PMID: 37905123 Free PMC article. Updated. Preprint.
-
Neuroinflammation in epileptogenesis: Insights and translational perspectives from new models of epilepsy.Epilepsia. 2017 Jul;58 Suppl 3(Suppl 3):39-47. doi: 10.1111/epi.13785. Epilepsia. 2017. PMID: 28675559 Free PMC article.
-
Characterization of Plaque-Sized Variants of Daniel's (DA) Strain in Theiler's Virus-Induced Epilepsy.Sci Rep. 2019 Mar 5;9(1):3444. doi: 10.1038/s41598-019-38967-z. Sci Rep. 2019. PMID: 30837498 Free PMC article.
-
Viral Triggers and Inflammatory Mechanisms in Pediatric Epilepsy.Mol Neurobiol. 2019 Mar;56(3):1897-1907. doi: 10.1007/s12035-018-1215-5. Epub 2018 Jul 5. Mol Neurobiol. 2019. PMID: 29978423 Free PMC article. Review.
-
Molecular Mechanisms in the Genesis of Seizures and Epilepsy Associated With Viral Infection.Front Mol Neurosci. 2022 May 9;15:870868. doi: 10.3389/fnmol.2022.870868. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35615063 Free PMC article. Review.
References
-
- Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. (1988) The risk of unprovoked seizures after encephalitis and meningitis. Neurology 38:1407–1410. - PubMed
-
- Annegers JF, Hauser WA, Coan SP, Rocca WA. (1998) A population-based study of seizures after traumatic brain injuries. N Engl J Med 338:20–24. - PubMed
-
- Barker-Haliski M, Friedman D, White HS, French JA. (2014a) How clinical development can, and should, inform translational science. Neuron 84:582–593. - PubMed
-
- Barker-Haliski M, Sills GJ, White HS. (2014b) What are the arguments for and against rational therapy for epilepsy? Adv Exp Med Biol 813:295–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

