Protein kinase C-dependent growth-associated protein 43 phosphorylation regulates gephyrin aggregation at developing GABAergic synapses
- PMID: 25755278
- PMCID: PMC4405633
- DOI: 10.1128/MCB.01332-14
Protein kinase C-dependent growth-associated protein 43 phosphorylation regulates gephyrin aggregation at developing GABAergic synapses
Abstract
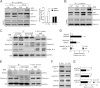
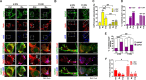
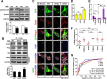
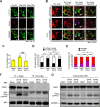
Growth-associated protein 43 (GAP43) is known to regulate axon growth, but whether it also plays a role in synaptogenesis remains unclear. Here, we found that GAP43 regulates the aggregation of gephyrin, a pivotal protein for clustering postsynaptic GABA(A) receptors (GABA(A)Rs), in developing cortical neurons. Pharmacological blockade of either protein kinase C (PKC) or neuronal activity increased both GAP43-gephyrin association and gephyrin misfolding-induced aggregation, suggesting the importance of PKC-dependent regulation of GABAergic synapses. Furthermore, we found that PKC phosphorylation-resistant GAP43(S41A), but not PKC phosphorylation-mimicking GAP43(S41D), interacted with cytosolic gephyrin to trigger gephyrin misfolding and its sequestration into aggresomes. In contrast, GAP43(S41D), but not GAP43(S41A), inhibited the physiological aggregation/clustering of gephyrin, reduced surface GABA(A)Rs under physiological conditions, and attenuated gephyrin misfolding under transient oxygen-glucose deprivation (tOGD) that mimics pathological neonatal hypoxia. Calcineurin-mediated GAP43 dephosphorylation that accompanied tOGD also led to GAP43-gephyrin association and gephyrin misfolding. Thus, PKC-dependent phosphorylation of GAP43 plays a critical role in regulating postsynaptic gephyrin aggregation in developing GABAergic synapses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
