Hepatitis B immunoglobulin prophylaxis after liver transplantation: experience in a tertiary transplant centre
- PMID: 25755562
- PMCID: PMC4284215
- DOI: 10.1016/j.jceh.2014.07.007
Hepatitis B immunoglobulin prophylaxis after liver transplantation: experience in a tertiary transplant centre
Abstract
Background: Prophylaxis with hepatitis B immunoglobulin (HBIG) and nucleoside analogs can prevent hepatitis B virus (HBV) recurrence after liver transplant (LT).
Aim: To determine the efficacy and cost of maintaining immunoprophylaxis with HBIG and hyperimmune plasma (HIP) for 6 months after LT.
Material & methods: The study included 22 HBV related LT recipients who were on entecavir and either HBIG or HIP for 6 months. Post transplant HBIG or HIP dose and cost incurred towards prophylaxis were noted. The cost of 200 IU of HBIG at the time of study was Rs 8250/- (US Dollars 135) and that of 2000 IU of HIP was Rs 8000/- (USD 130.7). The loading and maintenance costs at end of 6 months were compared between the two groups. Response to HBIG and HIP was assessed by checking for HBsAg reactivity, anti HBs titer response and HBV DNA viral load.
Statistical analysis: Median and range, Kruskal Wallis (KW) sign rank Sum Test and Correlation Coefficient (r2) was used for analysis.
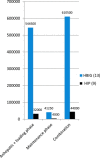
Results: Thirteen recipients received HBIG and 9 recipients HIP. The anti HBs response to HIP was significantly high compared to HBIG (KW Sign rank Sum test P < 0.05); titers remained high until the study period. Between 8 and 30 days, the titer achieved by both HBIG and HIP was similar (KW Sign rank Sum test not significant). Despite low anti HBs titer of <100 IU/L, none of the recipients on HBIG had HBsAg reactivity while 3 on HIP had transient HBsAg positivity. The total cost with HBIG was 13.9 times the cost of HIP.
Conclusion: HIP immunoprophylaxis in combination with entecavir achieves a high anti HBs titer at a significant low cost during anhepatic and loading phase. HBV reactivation rates with HBIG and HIP is low despite low anti HBs titer.
Keywords: ESLD, end-stage liver disease; HBIG, hepatitis B immunoglobulin; HBV recurrence; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIP, hyper-immune plasma; HIV, human immunodeficiency virus; Hepatitis B virus; KW, Kruskal Wallis; LT, liver transplantation; NA, nucleos(t)ide analogs; USD, US Dollars; anti HBs Ab, anti hepatitis B virus antibody; hepatitis B immunoglobulin; hyper-immune plasma; liver transplantation.
Figures
References
-
- Lok A.S. Prevention of recurrent hepatitis B post liver transplantation. Liver Transpl. 2002;8:867–873. - PubMed
-
- Roche B., Samuel D. Evolving strategies to prevent HBV recurrence. Liver Transpl. 2004;10:S74–S85. - PubMed
-
- Villami F.G. Prophylaxis with anti HBs immune globulins and nucleoside analogues after liver transplantation for HBV infection. J Hepatol. 2003;39:466–474. - PubMed
-
- Wong S.N., Chu C.J., Howell T. Low risk of hepatitis B virus recurrence after withdrawal of long term hepatitis B immunoglobulins in patients receiving maintenance nucleoside analogue therapy. Liver Transpl. 2007;13:374–381. - PubMed
-
- Neoumov N.V., Lopes A.R., Burra P. Randomised trial of lamivudine versus hepatitis B immunoglobulin for long term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol. 2001;34:888–894. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources