Safety of poly (ethylene glycol)-coated perfluorodecalin-filled poly (lactide-co-glycolide) microcapsules following intravenous administration of high amounts in rats
- PMID: 25756002
- PMCID: PMC4050377
- DOI: 10.1016/j.rinphs.2014.04.001
Safety of poly (ethylene glycol)-coated perfluorodecalin-filled poly (lactide-co-glycolide) microcapsules following intravenous administration of high amounts in rats
Abstract
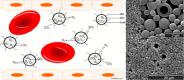
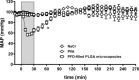
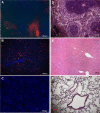
The host response against foreign materials designates the biocompatibility of intravenously administered microcapsules and thus, widely affects their potential for subsequent clinical use as artificial oxygen/drug carriers. Therefore, body distribution and systemic parameters, as well as markers of inflammation and indicators of organ damage were carefully evaluated after administration of short-chained poly (vinyl alcohol, (PVA)) solution or poly (ethylene glycol (PEG))-shielded perfluorodecalin-filled poly (d,l-lactide-co-glycolide, PFD-filled PLGA) microcapsules into Wistar rats. Whereas PVA infusion was well tolerated, all animals survived the selected dose of 1247 mg microcapsules/kg body weight but showed marked toxicity (increased enzyme activities, rising pro-inflammatory cytokines and complement factors) and developed a mild metabolic acidosis. The observed hypotension emerging immediately after start of capsule infusion was transient and mean arterial blood pressure restored to baseline within 70 min. Microcapsules accumulated in spleen and liver (but not in other organs) and partly occluded hepatic microcirculation reducing sinusoidal perfusion rate by about 20%. Intravenous infusion of high amounts of PFD-filled PLGA microcapsules was tolerated temporarily but associated with severe side effects such as hypotension and organ damage. Short-chained PVA displays excellent biocompatibility and thus, can be utilized as emulsifier for the preparation of drug carriers designed for intravenous use.
Keywords: ALAT, alanine aminotransferase; ANOVA, one-way analysis of variance; ASAT, aspartate aminotransferase; Artificial oxygen carriers; BE, base excess; Biocompatibility; Biodegradable microcapsules; C3, complement factor 3; C4a, complement factor 4a; CARPA, complement activation-related pseudoallergy; CK, creatine kinase; DAPI, 4',6-diamidin-2-phenylindol; FITC-dextran, fluorescein isothiocyanate-dextran 150,000; IFN-?, interferon-gamma; IL, interleukin; IVM, intravital microscopy; LDH, lactate dehydrogenase; MAP, mean arterial blood pressure; PEG, poly (ethylene glycol); PFD, perfluorodecalin; PLA); PLGA, poly (d,l-lactide-co-glycolide); PVA, poly (vinyl alcohol); Perfluorocarbon; Poly (lactic/glycolic) acid (PLGA; Poly (vinyl alcohol); TNF-a, tumor necrosis factor alpha; pO2, pCO2, oxygen and carbon dioxide partial pressures.
Figures



References
-
- Kuznetsova I.N. Perfluorocarbon emulsions: stability in vitro and in vivo (a review) Pharmaceutical Chemistry Journal. 2003;37:20–25.
-
- Bauer J., Zahres M., Zellermann A., Kirsch M., Petrat F., de Groot H., Mayer C. Perfluorocarbon-filled poly(lactide-co-gylcolide) nano- and microcapsules as artificial oxygen carriers for blood substitutes:a physico-chemical assessment. Journal of Microencapsulation. 2010;27:122–132. 20121485 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

