Evaluation of the synuclein-γ (SNCG) gene as a PPARγ target in murine adipocytes, dorsal root ganglia somatosensory neurons, and human adipose tissue
- PMID: 25756178
- PMCID: PMC4355072
- DOI: 10.1371/journal.pone.0115830
Evaluation of the synuclein-γ (SNCG) gene as a PPARγ target in murine adipocytes, dorsal root ganglia somatosensory neurons, and human adipose tissue
Abstract
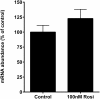
Recent evidence in adipocytes points to a role for synuclein-γ in metabolism and lipid droplet dynamics, but interestingly this factor is also robustly expressed in peripheral neurons. Specific regulation of the synuclein-γ gene (Sncg) by PPARγ requires further evaluation, especially in peripheral neurons, prompting us to test if Sncg is a bona fide PPARγ target in murine adipocytes and peripheral somatosensory neurons derived from the dorsal root ganglia (DRG). Sncg mRNA was decreased in 3T3-L1 adipocytes (~68%) by rosiglitazone, and this effect was diminished by the PPARγ antagonist T0070907. Chromatin immunoprecipitation experiments confirmed PPARγ protein binding at two promoter sequences of Sncg during 3T3-L1 adipogenesis. Rosiglitazone did not affect Sncg mRNA expression in murine cultured DRG neurons. In subcutaneous human WAT samples from two cohorts treated with pioglitazone (>11 wks), SNCG mRNA expression was reduced, albeit highly variable and most evident in type 2 diabetes. Leptin (Lep) expression, thought to be coordinately-regulated with Sncg based on correlations in human adipose tissue, was also reduced in 3T3-L1 adipocytes by rosiglitazone. However, Lep was unaffected by PPARγ antagonist, and the LXR agonist T0901317 significantly reduced Lep expression (~64%) while not impacting Sncg. The results support the concept that synuclein-γ shares some, but not all, gene regulators with leptin and is a PPARγ target in adipocytes but not DRG neurons. Regulation of synuclein-γ by cues such as PPARγ agonism in adipocytes is logical based on recent evidence for an important role for synuclein-γ in the maintenance and dynamics of adipocyte lipid droplets.
Conflict of interest statement
Figures




Similar articles
-
The DLK gene is a transcriptional target of PPARγ.Biochem J. 2011 Aug 15;438(1):93-101. doi: 10.1042/BJ20101840. Biochem J. 2011. PMID: 21585338
-
Gamma-synuclein is an adipocyte-neuron gene coordinately expressed with leptin and increased in human obesity.J Nutr. 2008 May;138(5):841-8. doi: 10.1093/jn/138.5.841. J Nutr. 2008. PMID: 18424589 Free PMC article.
-
Mechanisms of divergent effects of activated peroxisome proliferator-activated receptor-γ on mitochondrial citrate carrier expression in 3T3-L1 fibroblasts and mature adipocytes.Biochim Biophys Acta. 2013 Jun;1831(6):1027-36. doi: 10.1016/j.bbalip.2013.01.014. Epub 2013 Jan 28. Biochim Biophys Acta. 2013. PMID: 23370576
-
Role of PPARg2 transcription factor in thiazolidinedione-induced insulin sensitization.J Pharm Pharmacol. 2012 Feb;64(2):161-71. doi: 10.1111/j.2042-7158.2011.01366.x. Epub 2011 Oct 13. J Pharm Pharmacol. 2012. PMID: 22221092 Review.
-
Development of an In Vitro Screening Platform for the Identification of Partial PPARγ Agonists as a Source for Antidiabetic Lead Compounds.Molecules. 2018 Sep 22;23(10):2431. doi: 10.3390/molecules23102431. Molecules. 2018. PMID: 30248999 Free PMC article. Review.
Cited by
-
Profiling Cellular Processes in Adipose Tissue during Weight Loss Using Time Series Gene Expression.Genes (Basel). 2018 Oct 29;9(11):525. doi: 10.3390/genes9110525. Genes (Basel). 2018. PMID: 30380678 Free PMC article.
-
Effects of Dual Peroxisome Proliferator-Activated Receptors α and γ Activation in Two Rat Models of Neuropathic Pain.PPAR Res. 2019 Apr 17;2019:2630232. doi: 10.1155/2019/2630232. eCollection 2019. PPAR Res. 2019. PMID: 31139213 Free PMC article.
-
Lipids at the Crossroad of α-Synuclein Function and Dysfunction: Biological and Pathological Implications.Front Cell Neurosci. 2019 May 1;13:175. doi: 10.3389/fncel.2019.00175. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31118888 Free PMC article. Review.
-
Overexpression of synuclein-γ predicts lack of benefit from radiotherapy for breast cancer patients.BMC Cancer. 2016 Sep 5;16(1):717. doi: 10.1186/s12885-016-2750-y. BMC Cancer. 2016. PMID: 27595752 Free PMC article.
-
The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat.EMBO Mol Med. 2018 Mar;10(3):e8047. doi: 10.15252/emmm.201708047. EMBO Mol Med. 2018. PMID: 29343498 Free PMC article.
References
-
- van Baak MA (2001) The peripheral sympathetic nervous system in human obesity. Obes Rev 2: 3–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

