Irreversible endo-selective diels-alder reactions of substituted alkoxyfurans: a general synthesis of endo-cantharimides
- PMID: 25756502
- PMCID: PMC4406157
- DOI: 10.1002/chem.201406286
Irreversible endo-selective diels-alder reactions of substituted alkoxyfurans: a general synthesis of endo-cantharimides
Abstract
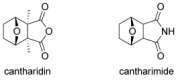
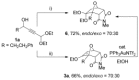
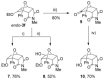
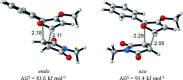
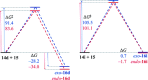
The [4+2] cycloaddition of 3-alkoxyfurans with N-substituted maleimides provides the first general route for preparing endo-cantharimides. Unlike the corresponding reaction with 3H furans, the reaction can tolerate a broad range of 2-substitued furans including alkyl, aromatic, and heteroaromatic groups. The cycloaddition products were converted into a range of cantharimide products with promising lead-like properties for medicinal chemistry programs. Furthermore, the electron-rich furans are shown to react with a variety of alternative dienophiles to generate 7-oxabicyclo[2.2.1]heptane derivatives under mild conditions. DFT calculations have been performed to rationalize the activation effect of the 3-alkoxy group on a furan Diels-Alder reaction.
Keywords: cantharimides; cycloadditions; dienes; furans; phthalimide.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures

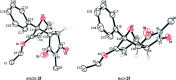
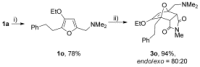
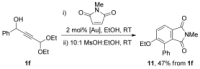
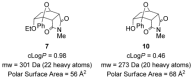

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

